The Oxidative Stress of Human Sperm Cryopreservation
- PMID: 40298642
- PMCID: PMC12024095
- DOI: 10.3390/antiox14040402
The Oxidative Stress of Human Sperm Cryopreservation
Abstract
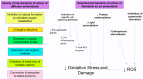
Due to their negligible cytoplasm and composition of the sperm plasmalemma, spermatozoa are particularly vulnerable to lipid peroxidative damage induced by reactive oxygen species (ROS). Most ROS are referred to as free radicals because they have unpaired electrons, causing them to scavenge electrons from atoms within tissues, resulting in oxidative damage to cellular components including cell membranes, intracellular organelles, and DNA. The potential consequences of oxidative stress include impaired sperm function, DNA fragmentation, and apoptosis. Understanding the mechanisms that mediate sperm damage during cryopreservation is key to the development of improved sperm freezing media formulations and methodology to mitigate its occurrence. Historically, elucidation of those mechanisms has proven largely elusive and is complicated by the positive role that ROS also play as messengers in redox signaling and the different pathways that mediate sperm DNA damage and apoptosis. More recently, oxidative stress has been revealed as the most likely suspect in cryopreservation-induced sperm DNA damage. This narrative review was intended to provide an in-depth analysis of the mechanisms involved and offer insight into possible improvements in sperm cryopreservation.
Keywords: cryopreservation; sperm DNA fragmentation; sperm cryopreservation; sperm plasmalemma; spermatozoa; vitrification.
Conflict of interest statement
The authors declare that they do not have any commercial or associative interests that represent a conflict of interest in connection with the work submitted. S.D.F. is also an employee of CooperSurgical Fertility Solutions, but the company had no knowledge of, or role in the design, execution, interpretation, or writing of this review article.
Figures
Similar articles
-
Effects of astaxanthin supplementation during vitrification and liquid nitrogen vapor freezing on motility, morphology, survival, reactive oxygen species (ROS), and DNA fragmentation of post-cryopreserved human sperm.JBRA Assist Reprod. 2024 Dec 3;28(4):611-617. doi: 10.5935/1518-0557.20240056. JBRA Assist Reprod. 2024. PMID: 39352311 Free PMC article.
-
Strategies to Minimize Various Stress-Related Freeze-Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa.Biopreserv Biobank. 2019 Dec;17(6):603-612. doi: 10.1089/bio.2019.0037. Epub 2019 Aug 20. Biopreserv Biobank. 2019. PMID: 31429586 Review.
-
Potent humanin analogue (HNG) protects human sperm from freeze-thaw-induced damage.Cryobiology. 2019 Jun;88:47-53. doi: 10.1016/j.cryobiol.2019.04.001. Epub 2019 Apr 6. Cryobiology. 2019. PMID: 30959025
-
Green tea extract as a cryoprotectant additive to preserve the motility and DNA integrity of human spermatozoa.Asian J Androl. 2021 Mar-Apr;23(2):150-156. doi: 10.4103/aja.aja_58_20. Asian J Androl. 2021. PMID: 33154201 Free PMC article.
-
Vitrification and conventional freezing methods in sperm cryopreservation: A systematic review and meta-analysis.Eur J Obstet Gynecol Reprod Biol. 2019 Feb;233:84-92. doi: 10.1016/j.ejogrb.2018.11.028. Epub 2018 Dec 14. Eur J Obstet Gynecol Reprod Biol. 2019. PMID: 30580229
Cited by
-
Engineered extracellular vesicles: a breakthrough approach to overcoming sperm cryopreservation challenges.Reprod Biol Endocrinol. 2025 May 21;23(1):75. doi: 10.1186/s12958-025-01407-x. Reprod Biol Endocrinol. 2025. PMID: 40399922 Free PMC article. Review.
References
-
- Banerjee A., Scarpa M., Pathak S., Burra P., Sturniolo G.C., Russo F.P., Murugesan R., D’Incá R. Inflammatory Bowel Disease Therapies Adversely Affect Fertility in Men- A Systematic Review and Meta-analysis. Endocr. Metab. Immune Disord. Drug Targets. 2019;19:959–974. doi: 10.2174/1871530319666190313112110. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources