Evaluation of Additive Neuroprotective Effect of Combination Therapy for Parkinson's Disease Using In Vitro Models
- PMID: 40298667
- PMCID: PMC12024093
- DOI: 10.3390/antiox14040396
Evaluation of Additive Neuroprotective Effect of Combination Therapy for Parkinson's Disease Using In Vitro Models
Abstract
Background: All the processes leading to neurodegeneration cannot be addressed with just one medication. Combinations of drugs affecting various disease mechanisms concurrently could demonstrate improved effect in slowing the course of Parkinson's disease (PD).
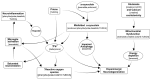
Objective: This was a drug-repurposing experiment designed to assess several combinations of nine drugs for possible added or synergistic efficacy using in vitro models of PD.
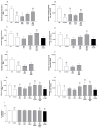
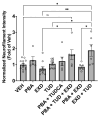
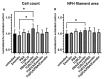
Methods: We evaluated 44 combinations of the nine medications (sodium phenylbutyrate, terazosin, exenatide, ambroxol, deferiprone, coenzyme-Q10, creatine, dasatinib and tauroursodeoxycholic acid) selected for their previously demonstrated evidence of their impact on different targets, showing neuroprotective properties in preclinical models of PD. We utilized wild-type induced pluripotent stem-cell-derived human dopaminergic neurons treated with 1-methyl-4-phenylpyridinium for initial screening. We retested some combinations using an idiopathic PD patient-derived induced pluripotent stem cell line and alpha-synuclein triplication line. We assessed anti-neuroinflammatory effects using human microglia cells. As metrics, we evaluated neurite length, number of branch points per mm2, the number of live neurons, neurofilament heavy chain and pro-inflammatory cytokines.
Results: We have identified four combinations of two to three drugs that showed an additive protective effect in some endpoints. Only the combination of sodium phenylbutyrate, exenatide and tauroursodeoxycholic acid showed improvement in four endpoints studied.
Conclusions: We demonstrated that some of the medications, used in combination, can exert an additive neuroprotective effect in preclinical models of PD that is superior to that of each of the compounds individually. This project can lead to the development of the first treatment for PD that can slow or prevent its progression.
Keywords: Parkinson’s disease; ROS; anti-inflammatory effects; combination therapy; drug repurposing; exenatide; iPSCs; in vitro models; sodium phenylbutyrate; tauroursodeoxycholic acid.
Conflict of interest statement
E.E. and F.S. are from Porsolt Research Laboratory where some of the experiments were performed. D.R. is from Prosoft Clinical and served as a statistician. These companies had no role in the design of the study; or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Mosharov E.V., Larsen K.E., Kanter E., Phillips K.A., Wilson K., Schmitz Y., Krantz D.E., Kobayashi K., Edwards R.H., Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. - DOI - PMC - PubMed
-
- Ryan S.D., Dolatabadi N., Chan S.F., Zhang X., Akhtar M.W., Parker J., Soldner F., Sunico C.R., Nagar S., Talantova M., et al. Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

