Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity
- PMID: 40298839
- PMCID: PMC12024330
- DOI: 10.3390/antiox14040463
Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity
Abstract
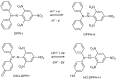
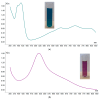
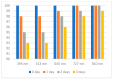
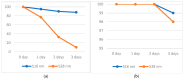
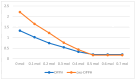
Numerous standardized methods for evaluating antioxidant capacity are available, some of the most used methods in this regard employ stable free radicals, like 2,2-diphenyl-1-picrilhydrazyl free radical (DPPH·) or 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid cation radical (ABTS·+). However, some challenges can arise in taking correct and reproducible measurements due to the well-known unspecific reactivity of these free radicals. In pursuit of improving and expanding such methods, in this work is proposed a highly intense colored zwitterionic derivative of the DPPH· free radical, as a replacement for DPPH· and ABTS·+ derivatives. A discussion and comparison of the recognized methods are presented, demonstrating the very good potential of this non-radical compound.
Keywords: ABTS; DPPH; TAC; antioxidant; betaine; radical; zwitterionic.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
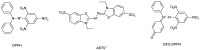


References
-
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. U.-Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. - DOI
-
- Sanchez-Moreno C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002;8:121. doi: 10.1177/1082013202008003770. - DOI
-
- Stoian M., Kuncser A., Neatu F., Florea M., Popa M., Voicu S., Chifiriuc M., Hanganu A., Anghel M., Tudose M. Green synthesis of aminated hyaluronic acid-based silver nanoparticles on modified titanium dioxide surface: Influence of size and chemical composition on their biological properties. Int. J. Biol. Macromol. 2023;253:127445. doi: 10.1016/j.ijbiomac.2023.127445. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

