Suspended Tissue Open Microfluidic Patterning (STOMP)
- PMID: 40298902
- PMCID: PMC12225008
- DOI: 10.1002/advs.202501148
Suspended Tissue Open Microfluidic Patterning (STOMP)
Abstract
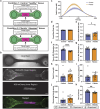
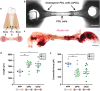
Free-standing tissue structures tethered between pillars are powerful mechanobiology tools for studying cell contraction. To model interfaces ubiquitous in natural tissues and upgrade existing single-region suspended constructs, we developed Suspended Tissue Open Microfluidic Patterning (STOMP), a method to create multi-regional suspended tissues. STOMP uses open microfluidics and capillary pinning to pattern subregions within free-standing tissues, facilitating the study of complex tissue interfaces, such as diseased-healthy boundaries (e.g., fibrotic-healthy) and tissue-type interfaces (e.g., bone-ligament). We observed altered contractile dynamics in fibrotic-healthy engineered heart tissues compared to single-region tissues and differing contractility in bone-ligament enthesis constructs compared to single-tissue periodontal ligament models. STOMP is a versatile platform - surface tension-driven patterning removes material requirements common with other patterning methods (e.g., shear-thinning, photopolymerizable) allowing tissue generation in multiple geometries with native extracellular matrices and advanced four-dimensional (4D) materials. STOMP combines the contractile functionality of suspended tissues with precise patterning, enabling dynamic and spatially controlled studies.
Keywords: hydrogel patterning; open microfluidics; suspended tissue; tissue engineering.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Multiple authors have filed patent 18/669,367 and 63/665,194 through the University of Washington on STOMP and related technology. ABT reports filing multiple patents through the University of Washington and receiving a gift to support research from Ionis Pharmaceuticals. EB and ABT have ownership in Seabright, LLC, which will advance new tools for diagnostics and clinical research. EB has ownership in Salus Discovery, LLC, and Tasso, Inc. and is employed by Tasso, Inc., Seabright, LLC, and the University of Washington. NJS has ownership in Stasys Medical Corporation, Inc. and has ownership and is a scientific advisor in Curi Bio, Inc. Technologies from Seabright, LLC, Salus Discovery, LLC, Tasso, Inc., Stasys Medical Corporation, Inc., and Curi Bio, Inc. are not included in this publication. EB is an inventor on multiple patents filed by Tasso, Inc., the University of Washington, and the University of Wisconsin Madison. The terms of this arrangement have been reviewed and approved by the University of Washington in accordance with its policies governing outside work and financial conflicts of interest in research. All other authors declare they have no competing interests.
Figures



Update of
-
Suspended Tissue Open Microfluidic Patterning (STOMP).bioRxiv [Preprint]. 2025 Mar 29:2024.10.04.616662. doi: 10.1101/2024.10.04.616662. bioRxiv. 2025. Update in: Adv Sci (Weinh). 2025 Jul;12(25):e2501148. doi: 10.1002/advs.202501148. PMID: 39416011 Free PMC article. Updated. Preprint.
References
-
- Park Y., Huh K. M., Kang S. W., Int. J. Mol. Sci. 2021, 22 , 2491.
MeSH terms
Grants and funding
- T32CA080416/NH/NIH HHS/United States
- F30HL158030/NH/NIH HHS/United States
- R35GM128648/NH/NIH HHS/United States
- F30 HL158030/HL/NHLBI NIH HHS/United States
- R35GM138036/NH/NIH HHS/United States
- P50AR065139/Senator Paul D. Wellstone Muscular Dystrophy Specialized Research Center - Seattle
- R90 DE023059/DE/NIDCR NIH HHS/United States
- 5TL1TR002318-08/NH/NIH HHS/United States
- TL1 TR002318/TR/NCATS NIH HHS/United States
- R01 HL149734/HL/NHLBI NIH HHS/United States
- R03DE029827/NH/NIH HHS/United States
- R35 GM128648/GM/NIGMS NIH HHS/United States
- R90DE023059/NH/NIH HHS/United States
- R03 DE029827/DE/NIDCR NIH HHS/United States
- R35 GM138036/GM/NIGMS NIH HHS/United States
- R01HL149734/NH/NIH HHS/United States
- T32 CA080416/CA/NCI NIH HHS/United States
- P50 AR065139/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
