Phase-1 study of vamotinib (PF-114), a 3rd generation BCR::ABL1 tyrosine kinase-inhibitor, in chronic myeloid leukaemia
- PMID: 40298994
- PMCID: PMC12141164
- DOI: 10.1007/s00277-025-06239-8
Phase-1 study of vamotinib (PF-114), a 3rd generation BCR::ABL1 tyrosine kinase-inhibitor, in chronic myeloid leukaemia
Abstract
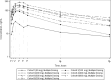
Vamotinib (PF-114) is a 3rd -generation, ATP-competitive oral tyrosine kinase inhibitor (TKI) active against wild-type and mutated BCR::ABL1 isoforms including BCR::ABL1T315I. We present final results of a phase-1 vamotinib dose-escalation study to identify maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) followed by expansion cohorts. 51 subjects with chronic myeloid leukaemia (CML) failing ≥ 1 2nd generation TKI or with BCR::ABL1T315I were enrolled. Subjects received vamotinib, 50-750 mg/d, continuously. Median exposure was 6 months (range, < 1-52 months). Median CML duration pre-study was 10 years (range, < 1-23 years). 27 subjects received ≥ 3 prior TKIs and 16 had BCR::ABL1T315I. The MTD was 600 mg with the Grade-3 psoriasis-like skin toxicity as the DLT. There were no vascular occlusive events nor deviations of ankle-brachial index. Complete haematologic response (CHR) was achieved in 14 of 30 subjects, major cytogenetic response (MCyR) in 14 of 44 subjects, complete cytogenetic response (CCyR) in 10 of 50 and major molecular response (MMR) in 7 of 51 subjects who did not have a CHR, MCyR, CCyR or MMR at enrollment. The best safety/efficacy dose was 300 mg with MCyR achieved in 6 of 7 subjects, CCyR in 5 of 9 and MMR in 4 of 9 subjects who did not have a MCyR, CCyR or MMR at enrollment. 5 of 16 subjects with BCR::ABL1T315I responded including 3 achieving a CHR, 3, a MCyR, and 1,a CCyR. 2 of 5 subjects failing ponatinib achieved a CHR. Vamotinib dose for further phase-3 study is 300 mg/d.
The trial is registered at clinicaltrials.gov. The registration number is NCT02885766, August 26, 2016.
Trial registration: ClinicalTrials.gov NCT02885766.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The protocol was approved by the ethics committee of the Ministry of Health of Russian Federation and by the participating clinical sites’ ethics committees. The study was conducted in accordance with the 1964 Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent. Consent for publication: Not applicable. Competing interests: AT – speaker: Novartis, Pfizer, R-Pharm, consultancy/advisory board: Novartis; OV: no competing interests to declare; EL has received fees for lecturing and expert opinion from Novartis, Pfizer, Sotex, Pharmstandard and Fusion Pharma; ES: no competing interests to declare; OS: no competing interests to declare; EC – speaker: Novartis, Pfizer, R-Pharm, consultancy: Ascentage Pharma; DS: no competing interests to declare; IN: no competing interests to declare; AP: speaker: Novartis, Alexpharm; AB: no competing interests to declare; NS: no competing interests to declare; Vasily Shuvaev received fees for lecturing and expert opinion from Novartis, Pfizer, Amgen, and AbbVie; IM: no competing interests to declare; FN was employed by Fusion Pharma; Veronika Shulgina: was employed by Fusion Pharma; AH: Honoraria and research funding (Novartis, Incyte); research funding (Bristol Myers Squibb, Pfizer, MSD); OO: Amgen, Incyte, Celgene, Roche, Fusion Pharma, Novartis: honoraria. Amgen, Incyte, and Celgene: research funding; JC: Consultancy (Pfizer, Takeda, Nerviano, Sun Pharma, Novartis, Biopath Holdings, Tigel); research funding (Ascentage, Novartis, Sun Pharma, Tern Pharma, Abbvie); membership on an entity’s board of directors or advisory committees (BioPath Holdings); RPG is a consultant to Antengene Biotech LLC, Ascentage Pharma Group and NexImmune Inc.; Medical Director, FFF Enterprises Inc.; A speaker for Janssen Pharma and Hengrui Pharma; Board of Directors: Russian Foundation for Cancer Research Support; and Scientific Advisory Boards, Nanexa AB and StemRad Ltd.; GC is employed by Fusion Pharma.
Figures
References
-
- Druker BJ, Guilhot F, O’Brien SG et al (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355:2408–2417. 10.1056/NEJMoa062867 - PubMed
-
- Kantarjian H, Shah NP, Hochhaus A et al (2010) Dasatinib versus Imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 362:2260–2270. 10.1056/nejmoa1002315 - PubMed
-
- Saglio G, Kim DW, Issaragrisil S et al (2010) Nilotinib versus Imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 362:2251–2259. 10.1056/nejmoa0912614 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous