Comprehensive Integrated Analysis Reveals the Spatiotemporal Microevolution of Cancer Cells in Patients with Bone-Metastatic Prostate Cancer
- PMID: 40299503
- PMCID: PMC12024866
- DOI: 10.3390/biomedicines13040909
Comprehensive Integrated Analysis Reveals the Spatiotemporal Microevolution of Cancer Cells in Patients with Bone-Metastatic Prostate Cancer
Abstract
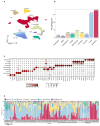
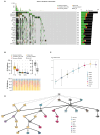
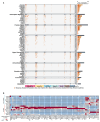
Background/Objectives: Bone metastasis is a frequent and life-threatening event in advanced cancers, affecting up to 70-85% of prostate cancer patients. Understanding the cellular and molecular mechanisms underlying bone metastasis is essential for developing targeted therapies. This study aimed to systematically characterize the heterogeneity and microenvironmental adaptation of prostate cancer bone metastases using single-cell transcriptomics. Methods: We integrated the largest single-cell transcriptome dataset to date, encompassing 124 samples from primary prostate tumors, various bone metastatic sites, and non-malignant tissues (e.g., benign prostatic hyperplasia, normal bone marrow). After quality control, 602,497 high-quality single-cell transcriptomes were analyzed. We employed unsupervised clustering, gene expression profiling, mutation analysis, and metabolic pathway reconstruction to characterize cancer cell subtypes and tumor microenvironmental remodeling. Results: Cancer epithelial cells dominated the tumor microenvironment but exhibited pronounced heterogeneity, posing challenges for conventional clustering methods. By integrating genetic and metabolic features, we revealed key evolutionary trajectories of epithelial cancer cells during metastasis. Notably, we identified a novel epithelial subpopulation, NEndoCs, characterized by unique differentiation patterns and distinct spatial distribution across metastatic niches. We also observed significant metabolic reprogramming and recurrent mutations linked to prostate-to-bone microenvironmental transitions. Conclusions: This study comprehensively elucidates the mutation patterns, metabolic reprogramming, and microenvironment adaptation mechanisms of bone metastasis in prostate cancer, providing key molecular targets and clinical strategies for the precise treatment of bone metastatic prostate cancer.
Keywords: bone metastasis; metabolism and cytokines; prostate cancer; single-cell RNA sequencing; spatiotemporal microevolution.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Bone metastasis in prostate cancer: Recurring mitochondrial DNA mutation reveals selective pressure exerted by the bone microenvironment.Bone. 2015 Sep;78:81-6. doi: 10.1016/j.bone.2015.04.046. Epub 2015 May 5. Bone. 2015. PMID: 25952970 Free PMC article.
-
Molecular insights into prostate cancer progression: the missing link of tumor microenvironment.J Urol. 2005 Jan;173(1):10-20. doi: 10.1097/01.ju.0000141582.15218.10. J Urol. 2005. PMID: 15592017 Review.
-
Unveiling the NEFH+ malignant cell subtype: Insights from single-cell RNA sequencing in prostate cancer progression and tumor microenvironment interactions.Front Immunol. 2024 Dec 20;15:1517679. doi: 10.3389/fimmu.2024.1517679. eCollection 2024. Front Immunol. 2024. PMID: 39759507 Free PMC article.
-
Single-cell omics traces the heterogeneity of prostate cancer cells and the tumor microenvironment.Cell Mol Biol Lett. 2023 May 9;28(1):38. doi: 10.1186/s11658-023-00450-z. Cell Mol Biol Lett. 2023. PMID: 37161356 Free PMC article. Review.
References
-
- Zi H., Liu M.-Y., Luo L.-S., Huang Q., Luo P.-C., Luan H.-H., Huang J., Wang D.-Q., Wang Y.-B., Zhang Y.-Y., et al. Global burden of benign prostatic hyperplasia, urinary tract infections, urolithiasis, bladder cancer, kidney cancer, and prostate cancer from 1990 to 2021. Mil. Med. Res. 2024;11:64. doi: 10.1186/s40779-024-00569-w. - DOI - PMC - PubMed
Grants and funding
- Grant No.2023YFC2507000 and No.2022YFE0200800/National Key R&D Program of China
- 2022I2M-1-008 and 2021-12M-1-015 and 2022-12M-2-002 and 2023-12M-QJ-006/the CAMS Initiative for Innovative Medicine
- 81972400 and 82202962/National Natural Science Foundation of China
- 2022-1-4021/The Capital Health Research and Development of Special Funding
LinkOut - more resources
Full Text Sources

