Identification and Functional Analysis of Cystathionine Beta-Synthase Gene Mutations in Chinese Families with Classical Homocystinuria
- PMID: 40299504
- PMCID: PMC12024673
- DOI: 10.3390/biomedicines13040919
Identification and Functional Analysis of Cystathionine Beta-Synthase Gene Mutations in Chinese Families with Classical Homocystinuria
Abstract
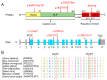
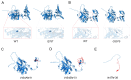
Background: Homocystinuria caused by cystathionine β-synthase (CBS) deficiency is the most common congenital disorder related to sulfur amino acid metabolism, manifested by neurological, vascular, and connective tissue involvement. Methods: This study analyzed the pathogenic gene and molecular mechanism of two classic homocystinuria families through whole exome sequencing and in vitro experiments including minigene assay and expression analysis. Results: Both probands presented with ectopia lentis, high myopia, and abnormally elevated homocysteine level, but one of them had more severe clinical manifestations, including general growth retardation, mild intellectual disability, and severe pectus excavatum. Their family members were phenotypically normal but presented slightly higher levels of homocysteine in plasma. Whole exome sequencing revealed that the two probands carried c.833T>C (p.Ile278Thr) and c.1359-1G>C, and c.919G>A (p.Gly307Ser) and c.131delT (p.Tle44Thrfs*38) compound heterozygous mutations in the CBS gene, respectively. Bioinformatics and in vitro functional analysis showed that the c.1359-1G>C mutation affects the normal splicing of CBS gene, resulting in the production of two abnormal transcripts and the production of two truncated proteins. One of the c.1359-1G>C splicing events (c.1359_1467del) and c.131delT (p.Tle44Thrfs*38) both lead to a significant decrease in CBS mRNA and protein levels. Conclusions: Accurate diagnosis of patients with homocystinuria is of great importance for timely and effective treatment, as well as for the provision of appropriate genetic counseling and prenatal diagnosis guidance to the affected families.
Keywords: CBS; RNA splicing; exome; homocystinuria; minigene; pathomechanism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Homocystinuria Caused by Cystathionine Beta-Synthase Deficiency.2004 Jan 15 [updated 2017 May 18]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2004 Jan 15 [updated 2017 May 18]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301697 Free Books & Documents. Review.
-
High prevalence of cerebral venous sinus thrombosis (CVST) as presentation of cystathionine beta-synthase deficiency in childhood: molecular and clinical findings of Turkish probands.Gene. 2014 Jan 25;534(2):197-203. doi: 10.1016/j.gene.2013.10.060. Epub 2013 Nov 6. Gene. 2014. PMID: 24211323
-
CBS mutations are good predictors for B6-responsiveness: A study based on the analysis of 35 Brazilian Classical Homocystinuria patients.Mol Genet Genomic Med. 2018 Mar;6(2):160-170. doi: 10.1002/mgg3.342. Epub 2018 Jan 20. Mol Genet Genomic Med. 2018. PMID: 29352562 Free PMC article.
-
Molecular and clinical characterisation of homocystinuria in two Austrian families with cystathionine beta-synthase deficiency.Acta Med Austriaca. 2001;28(5):145-51. doi: 10.1046/j.1563-2571.2001.01035.x. Acta Med Austriaca. 2001. PMID: 11774777
-
The Spectrum of Mutations of Homocystinuria in the MENA Region.Genes (Basel). 2020 Mar 20;11(3):330. doi: 10.3390/genes11030330. Genes (Basel). 2020. PMID: 32245022 Free PMC article. Review.
References
-
- Mudd S., Levy H., Kraus J.P. Disorders of transsulfuration. In: Scriver C., Beaudet A., Sly W., Valle D., Childs B., Kinzler K., Vogelstin B., editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY, USA: 2001. pp. 2007–2056.
Grants and funding
LinkOut - more resources
Full Text Sources

