Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors
- PMID: 40299508
- PMCID: PMC12024971
- DOI: 10.3390/biomedicines13040879
Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors
Abstract
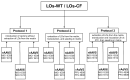
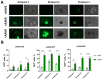
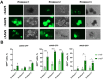
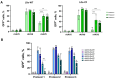
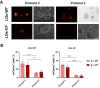
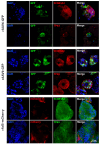
Background: Organoids are a valuable model for studying hereditary diseases such as cystic fibrosis (CF). Recombinant adenoviral (rAdV) and adeno-associated viral (rAAV) vectors are promising tools for CF gene therapy and genome editing. Objective: This study aims to determine the most efficient viral vector (rAdV5, rAAV serotypes 5, 6 and 9) and transduction protocol for delivering transgenes to lung organoids (LOs), providing a foundation for future CF gene therapy development. Methods: Three transduction protocols were used taking into account the specificities of LOs' cultivation in specific matrices, both with and without organoid extraction from the matrix. This work was carried out on organoids from a healthy donor (LOs-WT) and on a patient with cystic fibrosis (LOs-CF). Results: High transduction efficiency was observed with rAdV5 (30% cells), rAAV6 (>80% cells), and rAAV9 (>40% cells). rAdV5 and rAAV9 transduced basal and secretory cells with >90% efficiency. For rAAV9, Protocol 1 (without extraction of organoids from the matrix) showed lower transduction efficiency (33% for LOs-WT, 9% for LOs-CF), significantly lower than that of Protocols 2 (60% for LOs-WT, 59% for LOs-CF) and 3 (46% for LOs-WT, 35% for LOs-CF) with organoid extraction from the matrix (p < 0.005). Conclusions: rAdV5 and rAAV9 are the most promising vectors for the delivery of transgenes to basal and secretory cells in a lung organoid model, providing a solid foundation for CF gene therapy development.
Keywords: adeno-associated viral vectors; adenoviral vector; airway basal cells; cystic fibrosis; induced pluripotent stem cells; lung organoids.
Conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

