Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer-Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia
- PMID: 40299530
- PMCID: PMC12024546
- DOI: 10.3390/biomedicines13040892
Efficacy of Durvalumab Consolidation Therapy After Sequential Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer-Experience from the Daily Hospital of Clinic for Pulmonology, University Clinical Center of Serbia
Abstract
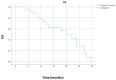
Background/Objectives: Patients with stage III non-small cell lung cancer represent a very heterogeneous group of patients. In the past, the standard of care for patients with inoperable stage III non-small cell lung cancer was concurrent or sequential radical radiotherapy and chemotherapy. But the progression-free survival was 8 months, and the 5-year overall survival rate was less than 20%. After the results of the PACIFIC study, the standard of care for this group of patients is chemoradiotherapy with durvalumab as consolidation therapy. The aim of our study was to evaluate the efficacy of consolidation durvalumab in a real-world setting after sequential CRT. Methods: We included 24 patients with unresectable stage III non-small cell lung cancer who did not progress after sequential chemoradiotherapy and who received durvalumab consolidation. Results: Median progression-free survival was 16 months, 95% CI (0.5-31.5), and median overall survival was 20 months, 95% CI (13.4-26.6 months). The twelve-month progression-free survival and overall survival rate were 55.1% and 68%, respectively, and the 18-month progression-free survival and overall survival rates were 44.1% and 56.5%, respectively. Conclusions: Durvalumab introduced a new era in the treatment of patients with unresectable stage III non-small cell lung cancer with a significantly prolonged 5-year overall survival rate. Our study is one of the few that investigated the efficacy of durvalumab in a real-world setting after sequential CRT. Our results showed that durvalumab is effective in patients who were treated with sequential CRT. However, the time between radiotherapy termination and the start of durvalumab should be shorter.
Keywords: OS; PFS; durvalumab; radiotherapy; real-world data; stage III NSCLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Curran W.J., Jr., Paulus R., Langer C.J., Komaki R., Lee J.S., Hauser S., Movsas B., Wasserman T., Rosenthal S.A., Gore E., et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. Erratum in J. Natl. Cancer Inst. 2012, 104, 79. - DOI - PMC - PubMed
-
- Ahn J.S., Ahn Y.C., Kim J.H., Lee C.G., Cho E.K., Lee K.C., Chen M., Kim D.W., Kim H.K., Min Y.J., et al. Multinational Randomized Phase III Trial with or without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J. Clin. Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. - DOI - PubMed
-
- Flentje M., Huber R.M., Engel-Riedel W., Andreas S., Kollmeier J., Staar S., Dickgreber N., Vaissiere N., De Almeida C., Edlich B., et al. A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther. Onkol. 2016;192:216–222. doi: 10.1007/s00066-016-0941-8. - DOI - PubMed
-
- Hanna N., Neubauer M., Yiannoutsos C., McGarry R., Arseneau J., Ansari R., Reynolds C., Govindan R., Melnyk A., Fisher W., et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J. Clin. Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous