Epac1 contributes to apremilast-mediated rescue of pemphigus autoantibody-induced loss of keratinocyte adhesion
- PMID: 40299565
- PMCID: PMC12128971
- DOI: 10.1172/jci.insight.187481
Epac1 contributes to apremilast-mediated rescue of pemphigus autoantibody-induced loss of keratinocyte adhesion
Abstract
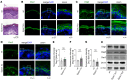
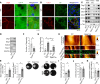
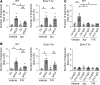
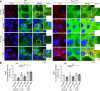
In the bullous autoimmune disease pemphigus vulgaris (PV), autoantibodies directed mainly against desmoglein 1 (Dsg1) and Dsg3 cause loss of desmosomal adhesion. We recently showed that intracellular cAMP increase by the phosphodiesterase 4 inhibitor apremilast was protective in different PV models. Thus, we here analyzed the involvement of the cAMP effector exchange factor directly activated by cAMP1 (Epac1). In Epac1-deficient mice pemphigus antibody-induced blistering was ameliorated in vivo while apremilast had no additional effect. Interestingly, augmented protein levels of Dsg1 and Dsg3 as well as increased Dsg1 mRNA levels and higher numbers of Dsg1- and Dsg3-dependent single-molecule interactions were detected in keratinocytes derived from Epac1-deficient mice. This was paralleled by stronger intercellular adhesion under baseline conditions and prevention of pemphigus autoantibody-induced loss of intercellular adhesion. However, the protective effect of apremilast against loss of intercellular adhesion in response to the pathogenic Dsg3 antibody AK23 was attenuated in Epac1-deficient keratinocytes. Similarly, the Epac1 inhibitor Esi09 protected keratinocytes from pemphigus antibody-induced loss of adhesion. Mechanistically, Epac1 deficiency resulted in lack of apremilast-induced Rap1 activation and phosphorylation of Pg at S665. Taken together, these data indicate that Epac1 is involved in the regulation of baseline and cAMP-mediated stabilization of keratinocyte adhesion.
Keywords: Autoimmune diseases; Cell biology; Cell migration/adhesion; Dermatology; Skin.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

