A gain-of-function mutation in ATP6V0A4 drives primary distal renal tubular alkalosis with enhanced V-ATPase activity
- PMID: 40299568
- PMCID: PMC12208546
- DOI: 10.1172/JCI188807
A gain-of-function mutation in ATP6V0A4 drives primary distal renal tubular alkalosis with enhanced V-ATPase activity
Abstract
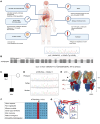
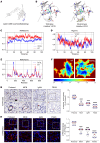
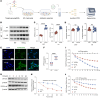
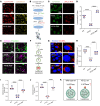
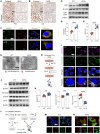
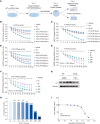
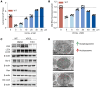
The ATP6V0A4 gene encodes the a4 subunit of vacuolar H+-ATPase (V-ATPase), which mediates hydrogen ion transport across the membrane. Previous studies have suggested that mutations in ATP6V0A4 consistently result in a loss of function, impairing the hydrogen ion transport efficacy of V-ATPase and leading to distal renal tubular acidosis and sensorineural hearing loss. Here, we identified a 32-year-old male patient and his father, both of whom harbored a heterozygous ATP6V0A4 p.V512L mutation and exhibited hypochloremic metabolic alkalosis, acidic urine, and hypokalemia. Through a series of protein structural analyses and functional experiments, the V512L mutation was confirmed as a gain-of-function mutation in the ATP6V0A4 gene. V512-a4 increased a4 subunit expression abundance by enhancing V512L-a4 stability and reducing its degradation, which in turn potentiated the capacity of V-ATPase to acidify the tubular lumen, leading to acidic urine and metabolic alkalosis. Through mutant V512L-a4 subunit structure-based virtual and experimental screening, we identified F351 (C25H26FN3O2S), a small-molecule inhibitor specifically targeting the V512L-a4 mutant. In conclusion, we identified a gain-of-function mutation in the ATP6V0A4 gene, broadening its phenotypic and mutational spectrum, and we provide valuable insights into potential therapeutic approaches for diseases associated with ATP6V0A4 mutations.
Keywords: Genetic diseases; Genetic variation; Genetics; Nephrology.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

