PAI-1 interaction with sortilin-related receptor 1 is required for lung fibrosis
- PMID: 40299646
- PMCID: PMC12220977
- DOI: 10.1172/jci.insight.186131
PAI-1 interaction with sortilin-related receptor 1 is required for lung fibrosis
Abstract
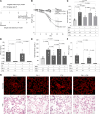
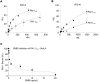
Mutation studies of plasminogen activator inhibitor 1 (PAI-1) have previously implied that PAI-1 promotes lung fibrosis via a vitronectin-dependent (VTN-dependent) mechanism. In the present study, employing 2 distinct murine fibrosis models and VTN-deficient mice, we found that VTN is not required for PAI-1 to drive lung scarring. This result suggested the existence of a profibrotic interaction involving the VTN-binding site on PAI-1 with an unidentified ligand. Using an unbiased proteomic approach, we identified sortilin-related receptor 1 (SorLA) as the most highly enriched PAI-1 binding partner in the fibrosing lung. Investigating the role of SorLA in pulmonary fibrosis demonstrated that deficiency of this protein protected against lung scarring in a murine model. We further found that SorLA is required for PAI-1 to promote scarring in mice, that both SorLA and PAI-1 protein levels are increased in human idiopathic pulmonary fibrosis (IPF) explants, and that these proteins are associated in IPF tissue. Finally, confocal microscopy showed that expression of SorLA in CHO cells increased cellular uptake of PAI-1, and these proteins colocalized in the cytoplasm. Together, these data elucidate a mechanism by which the potent profibrotic mediator PAI-1 drives lung fibrosis and implicate SorLA as a potential therapeutic target in IPF treatment.
Keywords: Aging; Fibrosis; Plasmin; Protein traffic; Pulmonology.
Figures




Update of
-
PAI-1 Interaction with Sortilin Related Receptor-1 is Required for Lung Fibrosis.bioRxiv [Preprint]. 2024 Aug 8:2024.08.06.606812. doi: 10.1101/2024.08.06.606812. bioRxiv. 2024. Update in: JCI Insight. 2025 Apr 29;10(11):e186131. doi: 10.1172/jci.insight.186131. PMID: 39211273 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

