INTERLINK-1: A Phase III, Randomized, Placebo-Controlled Study of Monalizumab plus Cetuximab in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
- PMID: 40300079
- PMCID: PMC12209814
- DOI: 10.1158/1078-0432.CCR-25-0073
INTERLINK-1: A Phase III, Randomized, Placebo-Controlled Study of Monalizumab plus Cetuximab in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
Abstract
Purpose: Treatment options for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) after failure of immune checkpoint inhibitor treatment and platinum-based chemotherapy are limited. Preliminary data suggested that monalizumab plus cetuximab had clinical activity in R/M HNSCC.
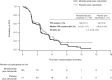
Patients and methods: INTERLINK-1 (NCT04590963) was a double-blind, phase III study. Participants with R/M HNSCC who had received immune checkpoint inhibitor therapy and progressed despite platinum-based chemotherapy were randomized 2:1 to monalizumab (750 mg, every 2 weeks) or placebo, plus cetuximab (400 mg/m2 loading dose, then 250 mg/m2, weekly). The primary endpoint was overall survival (OS) in participants with non-oropharyngeal cancer or human papillomavirus (HPV)-negative oropharyngeal cancer (HPV-unrelated analysis set). Secondary endpoints included progression-free survival and objective response rate.
Results: At data cutoff, 216 participants were randomized in the HPV-unrelated analysis set: 145 to monalizumab plus cetuximab and 71 to placebo plus cetuximab. Median OS was 8.8 months for monalizumab plus cetuximab versus 8.6 months for placebo plus cetuximab (HR, 1.00; 95% confidence interval, 0.66-1.54); median progression-free survival was 3.6 versus 3.8 months, respectively (HR, 1.11; 95% confidence interval, 0.79-1.57); and the objective response rate was 15.2% versus 23.9%, respectively. INTERLINK-1 was terminated after a preplanned interim analysis showed that futility criteria were met (predetermined futility HR >0.874). Grade 3/4 treatment-related adverse events were reported in 18.3% and 17.2% of participants treated in the monalizumab and placebo arms, respectively.
Conclusions: Monalizumab plus cetuximab did not improve OS compared with placebo plus cetuximab. The safety profile of the combination was consistent with safety observations for cetuximab monotherapy.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J. Fayette reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from Bristol Myers Squibb, MSD, Meru, Takeda, Seagen, Merck, Sanofi, and AstraZeneca outside the submitted work. L. Licitra reports personal fees from MSD IT, Merck Serono SpA Healthcare Professional, Merck Healthcare KGaA, GlaxoSmithKline, F. Hoffmann-La Roche Ltd., EMD Serono Research & Development Institute Inc., Boehringer Ingelheim International GmbH, Simon-Kucher & Partners Strategy & Marketing Consultants, Rgenta Therapeutics Inc., Alentis Therapeutics AG, MedImmune Limited, Simon-Kucher & Partners Italia Srl, Bristol Myers Squibb (K. Klinghammer), ALTIS Omnia Pharma Service Srl, Seagen International GmbH, Genmab US Inc., AbbVie Srl, Purple Biotech Ltd., AVEO Pharmaceuticals Inc., ALX Oncology Inc., Medscape LLC, and Bicara Therapeutics Inc.; nonfinancial support from TAE Life Science; and grants from Adlai Nortye, AstraZeneca, Bristol Myers Squibb, Debiopharm International SA, Eisai, Eli Lilly and Company, Exelixis, F. Hoffmann-La Roche Ltd., ISA Therapeutics, Kura Oncology, Merck Serono, MSD, Merck Sharp & Dome Corp, Nektar Therapeutics, Novartis, Regeneron, Sanofi, Syneos, Sun Pharmaceutical, Incyte Biosciences International Srl, Gilead Sciences, Inc., Genmab, and Merck Healthcare KGaA during the conduct of the study. K. Harrington reports grants and personal fees from AstraZeneca during the conduct of the study as well as personal fees from ALX Oncology, Beigene, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Merck Serono, Onchilles, Pfizer, and PsiVac; grants and personal fees from Boehringer Ingelheim, QBiotics, and Replimune; and grants, personal fees, and other support from MSD outside the submitted work. R. Haddad reports grants and personal fees from AstraZeneca during the conduct of the study as well as personal fees from Merck, GlaxoSmithKline, Hookipa, Johnson & Johnson, Nanobiotix, Genmab, PDS, Boehringer Ingelheim, EMD Serono, RAPT, Eisai, and AstraZeneca outside the submitted work. L.L. Siu reports personal fees from AstraZeneca and grants from AstraZeneca during the conduct of the study as well as personal fees from Amgen, Arvinas, Bristol Myers Squibb, Daiichi Sankyo, Gilead Sciences, GlaxoSmithKline, Incyte, LTZ Therapeutics, Marengo, Medicenna, Merck, Navire, Nerviano, Pangea, Relay Therapeutics, Roche/Genentech, Seattle Genetics, Tubulis, Voronoi, Treadwell Therapeutics, and Agios and grants from 23andMe, AbbVie, Amgen, Astellas, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, EMD Serono, Gilead Sciences, GlaxoSmithKline, Incyte, Legochem Biosciences, Loxo/Lilly, Medicenna, Merck, Novartis, Pfizer, Roche/Genentech, and Takara Bio outside the submitted work. M. Tahara reports personal fees from AstraZeneca during the conduct of the study as well as personal fees from Eisai, Novartis, MSD, Lilly, and Genmab and grants and personal fees from Ono Pharmaceutical and Bayer outside the submitted work. J.-P. Machiels reports other support from advisory board member or speaker with honoraria (managed by my institution): Pfizer, Roche, Bayer, Merck Serono, Boehringer, Bristol Myers Squibb, Novartis, Incyte, Cue Biopharma, ALX Oncology, iTeos Therapeutics, eTheRNA, Nektar Therapeutics, F-Star, Seagen, Genmab, Astellas, CureVac, MSD, GlaxoSmithKline, Merus, Ipsen, ANAVEON, and Bicara Therapeutics Inc., and other support from travel expenses from Amgen, Bristol Myers Squibb, Pfizer, MSD, Gilead, and Sanofi outside the submitted work. D. Rischin reports grants from AstraZeneca during the conduct of the study as well as grants from Regeneron, Merck (MSD), ALX Oncology, Erasca Inc., and Bicara Therapeutics outside the submitted work and is on the following uncompensated advisory boards and steering committees: Regeneron, Merck (MSD), GlaxoSmithKline, Eisai, and Bicara Therapeutics. T.Y. Seiwert reports personal fees from AstraZeneca during the conduct of the study as well as grants and personal fees from AstraZeneca, Regeneron, Pfizer, and Merck/MSD; nonfinancial support from IOBiotech; and personal fees from RAPT Therapeutics, Sanofi, Onchilles Pharma, and BioNTech/Syneos outside the submitted work. R.L. Ferris reports other support from AstraZeneca/Medimmune, Coherus Biosciences Inc., CureVac, MeiraGTX, Mirror Biological Sciences, Pfixer, Rakuten Medical Inc., Regeneron, and SirPant; personal fees from Bicara Therapeutics, EMD Serano, Everest Clinical Research, and Nanobiotix; and grants and other support from Merck, Merus N.V., and Novasenta outside the submitted work. U. Keilholz reports grants and personal fees from AstraZeneca and personal fees from Bristol Myers Squibb, Merck, MSD, and Novartis outside the submitted work. A. Psyrri reports other support from AstraZeneca during the conduct of the study as well as personal fees from Bristol Myers Squibb, Merus, AstraZeneca, MSD, and Merck Serono outside the submitted work. B. Keam reports grants from MSD Oncology, Ono Pharmaceutical, AstraZeneca, and Bayer outside the submitted work. P. Bossi reports personal fees from Merck, AstraZeneca, Pfizer, and Bicara Therapeutics; grants and personal fees from MSD and Regeneron; and grants from Beigene outside the submitted work. P.M.J. Clement reports other support from AstraZeneca during the conduct of the study, as well as grants from AstraZeneca and other support from MSD, Leo Pharma, Eisai, and Servier outside the submitted work. A. Mudunov reports grants from Hospital Lapino, Moscow, during the conduct of the study. S. Kasper reports personal fees from AstraZeneca during the conduct of the study as well as grants, personal fees, and other support from Amgen; grants and personal fees from Bristol Myers Squibb, Roche, and Lilly; personal fees from MSD, Takeda, Taiho, Servier, Daiichi Sankyo, Merck Healthcare, Pierre Fabre, and Incyte; and other support from Beigene outside the submitted work. M. Hwang reports other support from AstraZeneca during the conduct of the study. J. Blando reports other support from AstraZeneca outside the submitted work. O. Serrano reports other support from Cytel/AstraZeneca during the conduct of the study as well as other support from Cytel/AstraZeneca outside the submitted work; a patent for Cytel/AstraZeneca pending; and employment as an outsourced statistician with AstraZeneca. D. Ruscica reports employment with AstraZeneca. R.B. Cohen reports grants and personal fees from Innate Pharma and grants, personal fees, and nonfinancial support from AstraZeneca during the conduct of the study. No disclosures were reported by the other authors.
Figures


References
-
- Bhat GR, Hyole RG, Li J. Head and neck cancer: current challenges and future perspectives. Adv Cancer Res 2021;152:67–102. - PubMed
-
- Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol 2017;28:2386–98. - PubMed
-
- Ionna F, Bossi P, Guida A, Alberti A, Muto P, Salzano G, et al. Recurrent/metastatic squamous cell carcinoma of the head and neck: a big and intriguing challenge which may be resolved by integrated treatments combining locoregional and systemic therapies. Cancers (Basel) 2021;13:2371. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

