Insights into the bioluminescence systems of three sea pens (Cnidaria: Anthozoa): from de novo transcriptome analyses to biochemical assays
- PMID: 40300651
- PMCID: PMC12040472
- DOI: 10.1098/rsob.240262
Insights into the bioluminescence systems of three sea pens (Cnidaria: Anthozoa): from de novo transcriptome analyses to biochemical assays
Abstract
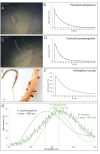
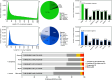
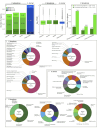
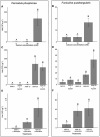
Bioluminescence is the production of visible light by living organisms. It occurs through the oxidation of luciferin substrates catalysed by luciferase enzymes. Auxiliary proteins, such as fluorescent proteins and luciferin-binding proteins, can modify the light emitted wavelength or stabilize reactive luciferin molecules, respectively. Additionally, calcium ions are crucial for the luminescence across various species. Despite the large phylogenetic distribution of bioluminescent organisms, only a few systems have been comprehensively studied. Notably, cnidarian species of the Renilla genus utilize a coelenterazine-dependent luciferase, a calcium-dependent coelenterazine-binding protein and a green fluorescent protein. We investigated the bioluminescence of three sea pen species: Pennatula phosphorea, Anthoptilum murrayi and Funiculina quadrangularis (Pennatuloidea, Anthozoa). Their light-emission spectra reveal peaks at 510, 513 and 485 nm, respectively. A coelenterazine-based reaction was demonstrated in all three species. Using transcriptome analyses, we identified transcripts coding for luciferases, green fluorescent proteins and coelenterazine-binding proteins for P. phosphorea and A. murrayi. Immunodetection confirmed the expression of luciferase in P. phosphorea and F. quadrangularis. We also expressed recombinant luciferase of A. murrayi, confirming its activity. We highlighted the role of calcium ions in bioluminescence, possibly associated with the mechanism of substrate release at the level of coelenterazine-binding proteins. The study proposes a model for anthozoan bioluminescence, offering new avenues for future ecological and functional research on these luminous organisms.
Keywords: Anthoptilidae; Funiculidae; Pennatulidae; bioluminescence; coelenterazine; luciferase; luciferin-binding protein; luminous system.
Conflict of interest statement
We declare we have no competing interests.
Figures






Similar articles
-
Etmopterus lantern sharks use coelenterazine as the substrate for their luciferin-luciferase bioluminescence system.Biochem Biophys Res Commun. 2021 Nov 5;577:139-145. doi: 10.1016/j.bbrc.2021.09.007. Epub 2021 Sep 6. Biochem Biophys Res Commun. 2021. PMID: 34517211
-
Coelenterazine-dependent luciferases.Biochemistry (Mosc). 2015 Jun;80(6):714-32. doi: 10.1134/S0006297915060073. Biochemistry (Mosc). 2015. PMID: 26531017 Review.
-
Coelenterazine-binding protein of Renilla muelleri: cDNA cloning, overexpression, and characterization as a substrate of luciferase.Photochem Photobiol Sci. 2008 Feb;7(2):189-96. doi: 10.1039/b713109g. Epub 2007 Dec 10. Photochem Photobiol Sci. 2008. PMID: 18264586
-
A puzzling homology: a brittle star using a putative cnidarian-type luciferase for bioluminescence.Open Biol. 2017 Apr;7(4):160300. doi: 10.1098/rsob.160300. Open Biol. 2017. PMID: 28381628 Free PMC article.
-
Acquisition of bioluminescent trait by non-luminous organisms from luminous organisms through various origins.Photochem Photobiol Sci. 2021 Nov;20(11):1547-1562. doi: 10.1007/s43630-021-00124-9. Epub 2021 Oct 29. Photochem Photobiol Sci. 2021. PMID: 34714534 Review.
References
-
- Shimomura O, Yampolsky I. 2019. Bioluminescence: chemical principles and methods, 3rd edn. Singapore: World Scientific Publishing.
-
- Delroisse J, Duchatelet L, Flammang P, Mallefet J. 2021. Leaving the dark side? Insights into the evolution of luciferases. Front. Mar. Sci. 8, 673620. (10.3389/fmars.2021.673620) - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

