Gut, bone, and muscle: the triad of osteosarcopenia in inflammatory bowel disease
- PMID: 40300749
- PMCID: PMC12332291
- DOI: 10.5217/ir.2024.00185
Gut, bone, and muscle: the triad of osteosarcopenia in inflammatory bowel disease
Abstract
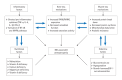
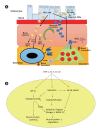
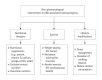
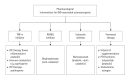
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions affecting the gastrointestinal tract that can lead to multiple systemic complications. Among these, osteosarcopenia has emerged as a significant concern, characterized by the concurrent deterioration of bone density and muscle mass, strength, and function. This dual deterioration significantly elevates the risk of falls and fractures, thereby exacerbating morbidity and diminishing quality of life. The pathogenesis of IBD-associated osteosarcopenia is multifactorial, with chronic intestinal inflammation serving as a central driver. Pro-inflammatory cytokines simultaneously disrupt bone homeostasis and muscle metabolism, creating a catabolic environment that impacts both tissues. Nutritional deficiencies, common in IBD due to malabsorption and decreased dietary intake, further compromise both bone mineralization and muscle protein synthesis. Management requires a comprehensive approach combining nutritional optimization, structured physical therapy, and lifestyle modifications. Pharmacological interventions integrate diseasespecific treatments with targeted therapies including vitamin D supplementation, hormonal treatments, and bisphosphonates when indicated. This review synthesizes current evidence regarding the prevalence, pathogenesis, and clinical impact of osteosarcopenia in IBD, highlighting areas requiring further investigation.
Keywords: Bone loss; Inflammatory bowel disease; Muscle loss; Osteoporosis; Sarcopenia.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Nutritional interventions for survivors of childhood cancer.Cochrane Database Syst Rev. 2016 Aug 22;2016(8):CD009678. doi: 10.1002/14651858.CD009678.pub2. Cochrane Database Syst Rev. 2016. PMID: 27545902 Free PMC article.
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
Investigating the Role of Nandrolone Decanoate in the Management of Osteosarcopenia in Postmenopausal Women: A Prospective Observational Study.Cureus. 2025 May 24;17(5):e84726. doi: 10.7759/cureus.84726. eCollection 2025 May. Cureus. 2025. PMID: 40551939 Free PMC article.
-
Patient education interventions for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2023 May 4;5(5):CD013854. doi: 10.1002/14651858.CD013854.pub2. Cochrane Database Syst Rev. 2023. PMID: 37172140 Free PMC article.
References
-
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. - PubMed
-
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. - PubMed
-
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. - PubMed
-
- Shen YH, Zhu H, Zhou L, et al. In inflammatory bowel disease and extraintestinal manifestations: what role does microbiome play? Eng Regen. 2023;4:337–348.
Publication types
LinkOut - more resources
Full Text Sources

