BAP1 Loss Affords Lipotoxicity Resistance in Uveal Melanoma
- PMID: 40300851
- PMCID: PMC12040534
- DOI: 10.1111/pcmr.70021
BAP1 Loss Affords Lipotoxicity Resistance in Uveal Melanoma
Abstract
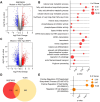
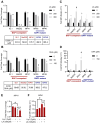
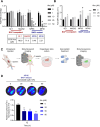
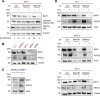
Uveal melanoma (UM) is an aggressive intraocular malignancy. Despite effective control of primary tumors, ~50% of UM patients develop metastases, with the liver being the predominant secondary site. BAP1 deficiency, present in ~80% of metastatic UM cases, is strongly associated with increased metastatic risk and poor prognosis. In silico analysis of UM patient samples suggests that reduced BAP1 is linked to enhanced expression of genes involved in fatty acid processing; therefore, we hypothesize that BAP1 deficiency primes UM cells for survival in the hepatic microenvironment by enhancing lipid tolerance and oxidative stress responses. Our findings demonstrate BAP1-mutant UM resist lipotoxicity, whereas BAP1-competent UM exhibit sensitivity due to lipid peroxide accumulation-a hallmark of ferroptotic-like stress, and a response that can be mitigated by ferroptosis inhibition. Using an ex vivo liver slice model, we found that disrupting lipid metabolism with atorvastatin, an HMG-CoA reductase inhibitor, reduced tumor burden of BAP1-mutant UM. Moreover, we demonstrate a positive correlation between BAP1 and an epigenetic regulator of lipid homeostasis, ASXL2. Notably, ASXL2 depletion in BAP1-competent UM phenocopies the lipotoxicity resistance observed in BAP1-mutant UM-an effect that may be mediated by altered PPAR expression. This study reveals a novel mechanism linking BAP1 expression to lipid sensitivity via ASXL2, providing insights into liver tropism and potential therapeutic avenues for metastatic uveal melanoma.
Keywords: ferroptosis; lipid metabolism; lipids; lipotoxicity; liver; liver microenvironment; metastatic uveal melanoma; uveal melanoma.
© 2025 The Author(s). Pigment Cell & Melanoma Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have nothing to report.
Figures

Similar articles
-
Slow proliferation of BAP1-deficient uveal melanoma cells is associated with reduced S6 signaling and resistance to nutrient stress.Sci Signal. 2024 Jun 11;17(840):eadn8376. doi: 10.1126/scisignal.adn8376. Epub 2024 Jun 11. Sci Signal. 2024. PMID: 38861613 Free PMC article.
-
ITGB2-ICAM1 axis promotes liver metastasis in BAP1-mutated uveal melanoma with retained hypoxia and ECM signatures.Cell Oncol (Dordr). 2024 Jun;47(3):951-965. doi: 10.1007/s13402-023-00908-4. Epub 2023 Dec 27. Cell Oncol (Dordr). 2024. PMID: 38150154
-
BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma.BMC Cancer. 2013 Aug 5;13:371. doi: 10.1186/1471-2407-13-371. BMC Cancer. 2013. PMID: 23915344 Free PMC article.
-
A comprehensive review of PRAME and BAP1 in melanoma: Genomic instability and immunotherapy targets.Cell Signal. 2024 Dec;124:111434. doi: 10.1016/j.cellsig.2024.111434. Epub 2024 Sep 24. Cell Signal. 2024. PMID: 39326690 Review.
-
[Uveal Melanoma: Molecular and Genetic Mechanisms of Development and Therapeutic Approaches].Mol Biol (Mosk). 2024 Mar-Apr;58(2):189-203. Mol Biol (Mosk). 2024. PMID: 39355878 Review. Russian.
References
-
- Ameer, F. , Scandiuzzi L., Hasnain S., Kalbacher H., and Zaidi N.. 2014. “De Novo Lipogenesis in Health and Disease.” Metabolism 63: 895–902. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

