Hermetia illucens-Derived Chitosan: A Promising Immunomodulatory Agent for Applications in Biomedical Fields
- PMID: 40300853
- PMCID: PMC12076490
- DOI: 10.1021/acs.biomac.5c00362
Hermetia illucens-Derived Chitosan: A Promising Immunomodulatory Agent for Applications in Biomedical Fields
Abstract
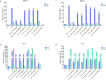
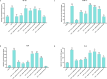
Chitosan, renowned for its important biological properties, is a valuable pharmaceutical excipient for different therapeutic approaches. Currently, the demand for the biopolymer on the market is growing, and, for this reason, it is important to biologically characterize the biopolymer produced from an alternative source to crustaceans, specifically the bioconverter insect Hermetia illucens. In this work, insect chitosan, yielded via heterogeneous and homogeneous deacetylation from larvae, pupal exuviae, and adults, was studied as an immunomodulatory agent. The inflammatory response of immortalized human keratinocyte cells was induced by Salmonella enterica subsp. enterica serovar Typhimurium lipopolysaccharide. After that, the ability of the biopolymer to reduce the expression of the pro-inflammatory cytokines IL-6, IL-8, IL-1α, and TNF-α was tested after 6 and 24 h of treatment. Insect chitosan samples effectively downregulated cytokine expression, with improved activity obtained from heterogeneous chitosan treatments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
A comprehensive characterization of Hermetia illucens derived chitosan produced through homogeneous deacetylation.Int J Biol Macromol. 2024 Jun;271(Pt 2):132669. doi: 10.1016/j.ijbiomac.2024.132669. Epub 2024 May 25. Int J Biol Macromol. 2024. PMID: 38801847
-
Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens.Sci Rep. 2022 May 16;12(1):8084. doi: 10.1038/s41598-022-12150-3. Sci Rep. 2022. PMID: 35577828 Free PMC article.
-
Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process.Sci Rep. 2022 Apr 22;12(1):6613. doi: 10.1038/s41598-022-10423-5. Sci Rep. 2022. PMID: 35459772 Free PMC article.
-
Harnessing the immunomodulatory potential of chitosan and its derivatives for advanced biomedical applications.Int J Biol Macromol. 2025 May;307(Pt 3):142055. doi: 10.1016/j.ijbiomac.2025.142055. Epub 2025 Mar 14. Int J Biol Macromol. 2025. PMID: 40090654 Review.
-
Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review.Res Vet Sci. 2019 Oct;126:68-82. doi: 10.1016/j.rvsc.2019.08.005. Epub 2019 Aug 7. Res Vet Sci. 2019. PMID: 31442715 Review.
Cited by
-
Physicochemical and Biological Properties of Quercetin-Loaded Low-Molecular-Weight Chitosan Nanoparticles Derived from Hermetia illucens Larvae and Crustacean Sources: A Comparative Study.Pharmaceutics. 2025 Aug 5;17(8):1016. doi: 10.3390/pharmaceutics17081016. Pharmaceutics. 2025. PMID: 40871037 Free PMC article.
References
-
- Jhundoo H. D.; Siefen T.; Liang A.; Schmidt C.; Lokhnauth J.; Béduneau A.; Pellequer Y.; Larsen C. C.; Lamprecht A. Anti-Inflammatory Activity of Chitosan and 5-Amino Salicylic Acid Combinations in Experimental Colitis. Pharmaceutics 2020, 12, 1038.10.3390/pharmaceutics12111038. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

