Inhibition of hepatic gluconeogenesis in type 2 diabetes by metformin: complementary role of nitric oxide
- PMID: 40300886
- PMCID: PMC12124709
- DOI: 10.4103/mgr.MEDGASRES-D-24-00100
Inhibition of hepatic gluconeogenesis in type 2 diabetes by metformin: complementary role of nitric oxide
Abstract
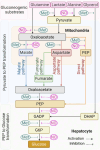
Metformin is the first-line treatment for type 2 diabetes mellitus. Type 2 diabetes mellitus is associated with decreased nitric oxide bioavailability, which has significant metabolic implications, including enhanced insulin secretion and peripheral glucose utilization. Similar to metformin, nitric oxide also inhibits hepatic glucose production, mainly by suppressing gluconeogenesis. This review explores the combined effects of metformin and nitric oxide on hepatic gluconeogenesis and proposes the potential of a hybrid metformin-nitric oxide drug for managing type 2 diabetes mellitus. Both metformin and nitric oxide inhibit gluconeogenesis through overlapping and distinct mechanisms. In hepatic gluconeogenesis, mitochondrial oxaloacetate is exported to the cytoplasm via various pathways, including the malate, direct, aspartate, and fumarate pathways. The effects of nitric oxide and metformin on the exportation of oxaloacetate are complementary; nitric oxide primarily inhibits the malate pathway, while metformin strongly inhibits the fumarate and aspartate pathways. Furthermore, metformin effectively blocks gluconeogenesis from lactate, glycerol, and glutamine, whereas nitric oxide mainly inhibits alanine-induced gluconeogenesis. Additionally, nitric oxide contributes to the adenosine monophosphate-activated protein kinase-dependent inhibition of gluconeogenesis induced by metformin. The combined use of metformin and nitric oxide offers the potential to mitigate common side effects. For example, lactic acidosis, a known side effect of metformin, is linked to nitric oxide deficiency, while the oxidative and nitrosative stress caused by nitric oxide could be counterbalanced by metformin's enhancement of glutathione. Metformin also amplifies nitric oxide -induced activation of adenosine monophosphate-activated protein kinase. In conclusion, a metformin-nitric oxide hybrid drug can benefit patients with type 2 diabetes mellitus by enhancing the inhibition of hepatic gluconeogenesis, decreasing the required dose of metformin for maintaining optimal glycemia, and lowering the incidence of metformin-associated lactic acidosis.
Keywords: glycemia; hepatocyte; hybrid; lactic acidosis; malate-aspartate shuttle; mitochondria; nitric oxide synthase; oxaloacetate; redox; tricarboxylic acid cycle.
Copyright © 2025 Medical Gas Research.
Conflict of interest statement
Figures



Similar articles
-
Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase.Nature. 2014 Jun 26;510(7506):542-6. doi: 10.1038/nature13270. Epub 2014 May 21. Nature. 2014. PMID: 24847880 Free PMC article.
-
Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo.Nat Med. 2018 Sep;24(9):1384-1394. doi: 10.1038/s41591-018-0125-4. Epub 2018 Jul 23. Nat Med. 2018. PMID: 30038219 Free PMC article.
-
Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism.J Biol Chem. 2019 Feb 22;294(8):2839-2853. doi: 10.1074/jbc.RA118.006670. Epub 2018 Dec 27. J Biol Chem. 2019. PMID: 30591586 Free PMC article.
-
Metformin--mode of action and clinical implications for diabetes and cancer.Nat Rev Endocrinol. 2014 Mar;10(3):143-56. doi: 10.1038/nrendo.2013.256. Epub 2014 Jan 7. Nat Rev Endocrinol. 2014. PMID: 24393785 Review.
-
Molecular action of metformin in hepatocytes: an updated insight.Curr Diabetes Rev. 2015;11(3):175-81. doi: 10.2174/1573399811666150325233108. Curr Diabetes Rev. 2015. PMID: 25808533 Review.
References
-
- DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. - PubMed
-
- International Diabetes Fedration . 10th. Brussels, Belgium: International Diabetes Federation; 2021. IDF Diabetes Atlas. http://www.diabetesatlas.org.
-
- Schernthaner G, Schernthaner GH. The right place for metformin today. Diabetes Res Clin Pract. 2020;159:107946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

