Composite mantle cell lymphoma and T-cell prolymphocytic leukemia: a case report
- PMID: 40301077
- PMCID: PMC12351245
- DOI: 10.3960/jslrt.24065
Composite mantle cell lymphoma and T-cell prolymphocytic leukemia: a case report
Abstract
We encountered a patient with composite mantle cell lymphoma (MCL) and T-cell prolymphocytic leukemia (T-PLL) who presented with inactive disease to active T-PLL over 8 years. A 71-year-old man was diagnosed with MCL with an atypical T-cell population showing CD2+, CD3-, CD4+, CD7+, CD8-, and CD25+; however, the cause of the T-cell population could not be determined at the first MCL diagnosis. When MCL relapsed approximately 8 years after the initial treatment, T-PLL was definitively diagnosed using the T-PLL International Study Group criteria. MCL and T-PLL were determined to coexist in the lymph nodes and bone marrow by histological or flowcytometry analysis. Retrospective flow cytometry and T-cell receptor-polymerase chain reaction analysis of the stored samples suggested that the T-cell population noted at the time of initial MCL diagnosis eight years earlier was the same clone of T-PLL and the progression from inactive disease to active disease of his T-PLL. To the best of our knowledge, this is the first report of a composite MCL and T-PLL.
Keywords: T-cell prolymphocytic leukemia; composite lymphoma; from inactive to active disease; mantle cell lymphoma.
Conflict of interest statement
Shigeru Kusumoto received research funding from Kyowa Kirin Co., Ltd., Bristol-Myers Squibb, Abbvie, Meiji Seika Pharma, and honoraria from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Janssen Pharmaceutical K.K., and Ono Pharmaceutical Co., Ltd..
Toko Saito received research funding from Meiji Seika Pharma. Co., IQVIA, Abbvie, AstraZeneca, Nippon-shinyaku.
Kazuhito Yamamoto received research funding from AbbVie, Astra-Zeneca, Bayer, Bristol-Myers Squibb/Celgene, Chugai, Eisai, IQIVA/Genmab, IQIVA/Incyte, MSD, Mundipharma, Nippon Shinyaku, Novartis, Ono, Otsuka, Solasia Pharma, SymBio, Takeda, Yakult, and Zenyaku, and consulting fees from Meiji Seika Pharma, and honoraria from AbbVie, Astellas, Astra-Zeneca, Bristol-Myers Squibb/Celgene, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Genmab, Gilead, Incyte, IQIVA/HUYA, Janssen, Kyowa Kirin, Meiji Seika Pharma, Micron/Daiichi Sankyo, MSD, Mundipharma, Nippon Kayaku, Nippon Shinyaku, Novartis, Ono, Otsuka, Pfizer, Sanofi, Sumitomo Pharma, SymBio, Takeda, and Yakult.
Figures
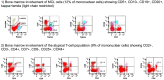


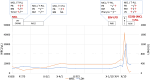


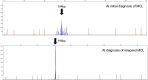
References
-
- Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991; 78: 259-263. - PubMed
-
- Garand R, Goasguen J, Brizard A, et al. Indolent course as a relatively frequent presentation in T-prolymphocytic leukaemia. Groupe Français d’Hématologie Cellulaire. Br J Haematol. 1998; 103: 488-494. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

