DUOX2 activation drives bacterial translocation and subclinical inflammation in IBD-associated dysbiosis
- PMID: 40301115
- PMCID: PMC12505107
- DOI: 10.1136/gutjnl-2024-334346
DUOX2 activation drives bacterial translocation and subclinical inflammation in IBD-associated dysbiosis
Abstract
Background: Inflammatory bowel diseases (IBDs) are characterised by dysbiosis and a leaky gut. The NADPH oxidase dual oxidase 2 (DUOX2) is upregulated in patients with IBD, yet its role in driving the disease remains unclear.
Objective: We interrogated the functional consequences of epithelial DUOX2 activity for the host and microbiome.
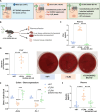
Design: DUOX2 function was studied in mice with epithelial-specific DUOX2 overactivation (vTLR4), inactivation (vTLR4 DUOXA IEC-KO) and wild-type controls. We assessed the effect of dysbiosis on DUOX2 signalling and intestinal permeability (FITC-dextran, serum zonulin, bacterial translocation) with germ-free (GF) mice engrafted with IBD or healthy microbiota. RNA sequencing of colonic mucosa and microbiota and faecal metabolomics were used to characterise the host-microbe interface. Mechanistic studies were conducted in mouse colonoids, IBD biopsies and patient serum samples.
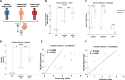
Results: DUOX2 activity increased permeability and bacterial translocation and induced subclinical inflammation in vTLR4 mice. GF vTLR4 mice had increased DUOX2 activity and permeability but no subclinical inflammation. In patients with IBD, DUOX2 expression was positively associated with plasma zonulin levels and negatively associated with ZO-1 expression. Engraftment of GF mice with IBD stool increased DUOX2 activity and triggered low-grade inflammation and permeability defects in mice. DUOX2 activity functionally altered the microbiome, reduced butyrate metabolism and promoted proinflammatory and pro-oncogenic bacterial metabolites. Butyrate and histone deacetylase (HDAC) inhibitors blocked DUOX2 activation and reversed its effects.
Conclusions: Elevated DUOX2 signalling contributes to epithelial barrier dysfunction, microbiome alterations and subclinical inflammation. Butyrate and HDAC inhibitors reversed these effects, indicating that DUOX2 may be a therapeutic target in IBD.
Keywords: INFLAMMATORY BOWEL DISEASE; INTESTINAL ENZYMES; INTESTINAL EPITHELIUM; INTESTINAL MICROBIOLOGY; REACTIVE OXYGEN SPECIES.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MTA serves as a consultant or on the advisory board of the following companies: AbbVie, Alimentiv, Amgen, Bristol Myers Squibb, Eli Lilly and Company, Genetech, Gilead Sciences, Janssen Pharmaceuticals, Pfizer Pharmaceutical, Takeda Pharmaceuticals, and UCB Pharma. CJ is an associate editor at Gut. All other authors have no conflicts of interest to declare.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
