Degradation rather than disassembly of necrotic debris is essential to enhance recovery after acute liver injury
- PMID: 40301163
- PMCID: PMC12040799
- DOI: 10.1007/s00018-025-05720-x
Degradation rather than disassembly of necrotic debris is essential to enhance recovery after acute liver injury
Abstract
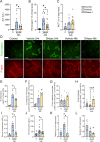
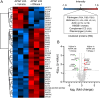
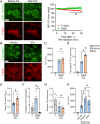
Necrotic cell death causes loss of membrane integrity, release of intracellular contents and deposition of necrotic cell debris. Effective clearance of this debris is crucial for resolving inflammation and promoting tissue recovery. While leukocyte phagocytosis plays a major role, soluble factors in the bloodstream also contribute to debris removal. Our study examined whether enzymatic degradation or disassembly of necrotic debris enhances clearance and improves outcomes in a mouse model of drug-induced liver injury. Using intravital microscopy and on-tissue spatially-resolved microproteomics, we demonstrated that necrotic debris is more complex than anticipated, containing DNA, filamentous actin, histones, complement C3, fibrin(ogen) and plasmin(ogen), among many other components. DNase 1 treatment facilitated recovery significantly by enhancing the clearance of DNA from necrotic areas, reducing circulating nucleosomes and actin, and lowering the associated inflammatory response. However, its effect on actin and other damage-associated molecular patterns in necrotic regions was limited. Treatment with short synthetic peptides, specifically 20-amino acid-long positively charged poly L-lysine (PLK) and negatively charged poly L-glutamic acid (PLE), which displace histones from debris in vitro, did not inhibit liver injury or promote recovery. Moreover, activating plasmin to disrupt fibrin encapsulation via tissue plasminogen activator (tPa) led to increased circulating actin levels and worsening of injury parameters. These findings suggest that fibrin encapsulation is important for containing necrotic debris and that enzymatic degradation of necrotic debris is a more effective strategy to enhance tissue recovery than targeting debris disassembly.
Keywords: Cell death; DNase; Fibrinolysis; Necrotic cell debris; Peptide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The animal study was approved by the Ethical Committee for Animal Experiments from KU Leuven (registry number: P128/2021). The study was conducted in accordance with the local legislation and institutional requirements. Conflict of interest: The authors have no conflicts to disclose.
Figures


References
-
- Ahrens S, Zelenay S, Sancho D et al (2012) F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36:635–645. 10.1016/j.immuni.2012.03.008 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

