Enrichment of human IgA-coated bacterial vesicles in ulcerative colitis as a driver of inflammation
- PMID: 40301356
- PMCID: PMC12041585
- DOI: 10.1038/s41467-025-59354-5
Enrichment of human IgA-coated bacterial vesicles in ulcerative colitis as a driver of inflammation
Abstract
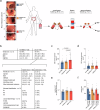
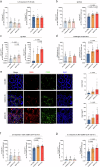
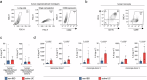
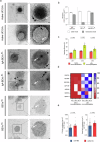
The gut microbiome contributes to chronic inflammatory responses in ulcerative colitis (UC), but molecular mechanisms and disease-relevant effectors remain unclear. Here we analyze the pro-inflammatory properties of colonic fluid obtained during colonoscopy from UC and control patients. In patients with UC, we find that the pelletable effector fraction is composed mostly of bacterial extracellular vesicles (BEVs) that exhibit high IgA-levels and incite strong pro-inflammatory responses in IgA receptor-positive (CD89+) immune cells. Biopsy analyses reveal higher infiltration of CD89+ immune cells in the colonic mucosa from patients with UC than control individuals. Further studies show that IgA-coated BEVs, but not host-derived vesicles nor soluble IgA, are potent activators of pro-inflammatory responses in CD89+ cells. IgA-coated BEVs also exacerbate intestinal inflammation in a dextran sodium sulfate colitis model using transgenic mice expressing human CD89. Our data thus implicate a link between IgA-coated BEVs and intestinal inflammation via CD89+ immune cells, and also hint a potential new therapeutic target for UC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
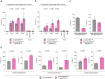
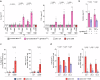

References
-
- Torres, J., Billioud, V., Sachar, D. B., Peyrin-Biroulet, L. & Colombel, J. F. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm. Bowel Dis.18, 1356–1363 (2012). - PubMed
-
- Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Prim.6, 74 (2020). - PubMed
-
- Le Berre, C., Honap, S. & Peyrin-Biroulet, L. Ulcerative colitis. Lancet402, 571–584 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

