Ophthalmological manifestations and plasma markers of inflammation in Ebola survivors in post-treatment era
- PMID: 40301432
- PMCID: PMC12041595
- DOI: 10.1038/s41598-025-96256-4
Ophthalmological manifestations and plasma markers of inflammation in Ebola survivors in post-treatment era
Abstract
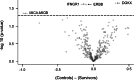
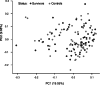
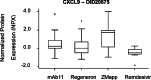
This study aimed to characterize ophthalmological manifestations and associated inflammatory markers in EVD survivors in post-treatment era. Case-control study of ophthalmological manifestations and plasma inflammatory biomarker profile in EVD survivors (n = 120) from the 2018-2020 outbreak in DRC, their gender- and age-matched close contacts (n = 120) and non-contact (healthy) controls (n = 120). Expressions of inflammatory markers were assessed using the Olink Explore 384 Assay and compared across study groups before and after stratification by treatment with monoclonal antibodies (mAB114, ZMapp, or Regeneron) or antiviral drug (Remdesivir). Protein profiling was carried out using the Olink statistical package. Mean age (years) was comparable among survivors (29.7 ± 10.6), close contacts (28.9 ± 11.1) and non-contact controls (29.3 ± 10.6) (p = 0.85). Mean time from disease onset to clinical assessment was 3.5 ± 0.5 (2.5-4.2) years in survivors. Optic neuropathy was more common in survivors (6.7%) than in close contacts (0.8%) and non-contacts (0.0%) (p = 0.003). Survivors with optic neuropathy had significantly worse visual acuity in both eyes than those without optic neuropathy (all p < 0.001). Clinical evidence of past anterior uveitis was observed in 2.5% of survivors, 2.9% of close contacts, and 1.8% of healthy controls (p = 0.86). Plasma circulating DGKZ, INFGR1, ERBB3, and MICA-MICB showed differential expression patterns between survivors and controls (all p < 0.05). However, no clear separation could be detected on principal component analysis of multiplexed proteomic data between survivor and control samples. Three proteins (ITM2A, CLEC4D, NCLN) were differentially expressed and related to optic neuropathy. The comparison between treatment groups revealed a trend toward lower protein inflammatory markers in survivors treated with Remdesivir than those treated with monoclonal antibodies. We conclude that in treated EVD survivors, optic neuropathy was the only neuro-ophthalmologic abnormality. Uveitis was far less frequent than reported in West African cohorts. ITM2A, CLEC4D, and NCLN were differentially expressed in EVD survivors with optic neuropathy long after the acute phase of the infection. The true meaning of these findings will need further investigations.
Keywords: Ebola treatment; Ebola virus disease; Inflammatory markers; Optic neuropathy; Uveitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Centers for Disease, C. Prevention. Update: outbreak of Ebola viral hemorrhagic fever–Zaire, 1995. MMWR Morb Mortal. Wkly. Rep.44, 468–469 (1995). - PubMed
-
- Centers for Disease Control and Prevention. Ebola outbreak history. (2024). Available at https://www.cdc.gov/ebola/outbreaks/index.html. Accessed August 1.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

