BMP-2 mRNA-transfected BMSCs promote superior calvarial bone regeneration
- PMID: 40301548
- PMCID: PMC12041208
- DOI: 10.1038/s41598-025-99979-6
BMP-2 mRNA-transfected BMSCs promote superior calvarial bone regeneration
Abstract
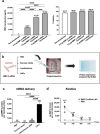
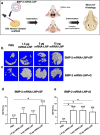
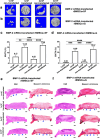
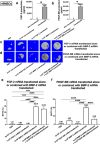
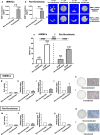
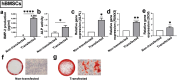
Large critical-size bone defects in the oral and craniofacial region are difficult to regenerate. We evaluated the effectiveness of mRNA encoding bone morphogenic protein-2 (BMP-2) in enhancing bone regeneration using a rat calvarial defect model. Two delivery approaches were investigated: (1) in vivo application of BMP-2 mRNA encapsulated in lipid nanoparticles incorporated in a scaffold, and (2) application of ex vivo BMP-2 mRNA-transfected rat bone marrow mesenchymal stem cells (rBMSCs), loaded on a scaffold and implanted into calvarial defects. The direct application of BMP-2 mRNA encapsulated in lipid nanoparticles improved bone regeneration as indicated by micro-computed tomography analysis. The enhancement was even more pronounced with ex vivo transfected rBMSCs. rBMSCs transfected with FGF-2 mRNA did not improve bone regeneration, either alone or combined with BMP-2 mRNA-transfected rBMSCs. Similarly, PDGF-BB mRNA-transfected rBMSCs failed to enhance bone regeneration alone and notably suppressed BMP-2 mRNA-transfected rBMSCs' effects. Interestingly, BMP-2 mRNA-transfected rat fibroblasts showed comparable bone regeneration to transfected rBMSCs. Osteogenic differentiation was absent in BMP-2 mRNA-transfected rBMSCs, implying that they may primarily serve as a source of translated BMP-2 for bone regeneration rather than undergoing osteogenic differentiation. These findings highlight the translational potential of BMP-2 mRNA for bone regeneration, particularly in oral and craniofacial applications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

