Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis
- PMID: 40301561
- PMCID: PMC12092284
- DOI: 10.1038/s41591-025-03678-8
Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis
Abstract
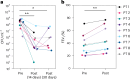
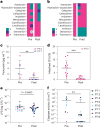
Bacteriophage (phage) therapy, which uses lytic viruses as antimicrobials, is a potential strategy to address the antimicrobial resistance crisis. Cystic fibrosis, a disease complicated by recurrent Pseudomonas aeruginosa pulmonary infections, is an example of the clinical impact of antimicrobial resistance. Here, using a personalized phage therapy strategy that selects phages for a predicted evolutionary trade-off, nine adults with cystic fibrosis (eight women and one man) of median age 32 (range 22-46) years were treated with phages on a compassionate basis because their clinical course was complicated by multidrug-resistant or pan-drug-resistant Pseudomonas that was refractory to prior courses of standard antibiotics. The individuals received a nebulized cocktail or single-phage therapy without adverse events. Five to 18 days after phage therapy, sputum Pseudomonas decreased by a median of 104 CFU ml-1, or a mean difference of 102 CFU ml-1 (P = 0.006, two-way analysis of variance with Dunnett's multiple-comparisons test), without altering sputum microbiome, and an analysis of sputum Pseudomonas showed evidence of trade-offs that decreased antibiotic resistance or bacterial virulence. In addition, an improvement of 6% (median) and 8% (mean) predicted FEV1 was observed 21-35 days after phage therapy (P = 0.004, Wilcoxon signed-rank t-test), which may reflect the combined effects of decreased bacterial sputum density and phage-driven trade-offs. These results show that a personalized, nebulized phage therapy trade-off strategy may affect clinical and microbiologic endpoints, which must be evaluated in larger clinical trials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The following authors declare no competing interests: G.L.S., A.C.V., M.M., I.M.O., Y.S., S.W., C.N.G., B.I.K., G.R., Z.M.H., C.J.B., J.S., J.S.T., C.R.A., N.C., S.K.J., R.J., A.K., B.S.Q., J.M.R., C.C.T., Q.-A.M., V.S. and J.L.K. The following authors declare a competing interest: B.K.C., K.E.K. and P.E.T. for US Provisional Patent Application No. 62/844,515, filed 7 May 2019, and US Provisional Patent Application No. 63/017,369, filed 29 April 2020, for bacteriophages (OMKO1, LPS-5 and TIVP-H6) that have been studied in this project.
Figures






References
-
- O’Neill, J. in Review on Antimicrobial Resistance 1–14 (HM Government & Wellcome Trust, 2014).
-
- Kortright, K. E., Chan, B. K., Koff, J. L. & Turner, P. E. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe25, 219–232 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

