Efficacy and safety of Mirikizumab for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials
- PMID: 40301737
- PMCID: PMC12039255
- DOI: 10.1186/s12876-025-03627-2
Efficacy and safety of Mirikizumab for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials
Abstract
Ulcerative colitis (UC) is a widespread incurable chronic inflammation of the colon mucosa. Currently, oral small-molecule medications targeting Janus kinase or sphingosine-1-phosphate and monoclonal antibodies to TNF-α,α4β7 integrins and Ustekinumab are the lines of treatment for UC. Up to 50% of patients either do not react to initial treatment or lose response over time, emphasizing the need for innovative treatment. Mirikizumab, a humanized IgG4-variant monoclonal antibody, binds to subunit p19 of interleukin-23. This systematic review aims to evaluate Mirikizumab compared to placebo in treating moderate-to-severe active UC. Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and using the Population, Intervention, Comparison, Outcome, Study design (PICOS) model for inclusion and exclusion criteria, we systematically reviewed the literature. Our inclusion criteria encompassed randomized controlled trials assessing Mirikizumab efficacy in treating UC across demographics. We employed the Cochrane Risk of Bias tool (RoB1) to investigate bias within included studies across its seven domains. The statistical analysis was conducted using Review Manager Version 5 software. Four studies were included, comparing patients treated with mirikizumab to placebo groups. All doses of mirikizumab administered intravenously demonstrated clinical remission, specifically, the 200 mg and 300 mg doses showed significant efficacy, with risk ratios of 4.74 (95% CI [1.43, 15.69]) and 1.82 (95% CI [1.33, 2.50]), respectively. During the maintenance phase of extension trials, symptoms subsided with a subcutaneous 200 mg dose (RR = 1.46, 95% CI [0.47, 4.51], P = 0.51). To conclude, mirikizumab demonstrates significant efficacy in treating UC, substaintially improving clinical, endoscopic, and histological outcomes.
Keywords: IL-23 inhibitor; Meta-analysis; Mirikizumab; Ulcerative colitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Does not apply. There was no involvement of new human participants in this study. Consent for publication: Does not apply. There was no involvement of new human participants in this study. Competing interests: The authors declare no competing interests.
Figures
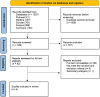
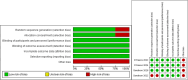





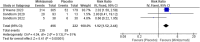




References
-
- Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020;35:380–9. - PubMed
-
- Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet Lond Engl. 2023;402:571–84. - PubMed
-
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet Lond Engl. 2012;380:1606–19. - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet Lond Engl. 2007;369:1641–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

