Drug-drug interaction among elderly patients in Africa: a systematic review and meta-analysis
- PMID: 40301898
- PMCID: PMC12039052
- DOI: 10.1186/s40360-025-00926-y
Drug-drug interaction among elderly patients in Africa: a systematic review and meta-analysis
Abstract
Background: Elderly patients are at a heightened risk of drug-drug interactions due to their high prevalence of comorbidities, polypharmacy, and age-related physiological changes that alter drug metabolism and excretion. In Africa, these risks are compounded by unique healthcare challenges, including limited access to diagnostic tools, and high burdens of communicable diseases. The aim of this study is to estimate the prevalence of drug-drug interactions and its associated factors among elderly patients in Africa.
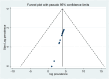
Methods: Relevant research articles were identified from databases such as HINARI, Science Direct, Embase, PubMed/MEDLINE, Google Scholar, and Research Gate. Data were extracted via a Microsoft Excel spreadsheet and analyzed via STATA version 11.0. Egger regression tests and funnel plot analysis were used to check for publication bias, and the I2 statistic was used to evaluate statistical heterogeneity. Sensitivity and subgroup analyses were also conducted to identify potential causes of heterogeneity.
Results: Fifteen articles were analyzed, and a total of 5651 potential drug-drug interactions (pDDIs) were identified in 1952 patients, resulting in an average of 2.89 pDDIs per patient. The overall prevalence of pDDIs among elderly patients was 52.53% (95% confidence interval (CI): 35.40, 69.66). However, the prevalence of pDDIs ranged widely from 2.8 to 90.1%. When the severity of the interactions was considered, the prevalence of pDDIs was 20.59%, 69.4%, 34.32% and 1.59% for major, moderate, minor, and contraindicated DDIs, respectively. Polypharmacy, long hospital stays, hypertension and diabetes mellitus were identified as factors associated with pDDIs among elderly patients in Africa.
Conclusion: DDIs are prevalent among elderly patients in Africa and are often associated with polypharmacy, prolonged hospitalizations, and the presence of chronic comorbidities, particularly hypertension and diabetes mellitus. Moderate-severity interactions were the most prevalent DDIs. The study suggests addressing this issue requires targeted interventions, including improved pharmacovigilance, enhanced prescribing practices, and integration of DDI risk assessment into routine clinical care. The study also suggests that the database itself could have modified the DDI prevalence rate. As a result, a single DDI identification database needs to be authorized; otherwise, clinical knowledge should be taken in to account when interpreting the information obtained.
Keywords: Africa; Drug-drug interaction; Elderly; Meta-analysis; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Hadjibabaie M, Badri S, Ataei S, Moslehi AH, Karimzadeh I, Ghavamzadeh A. Potential drug–drug interactions at a referral hematology–oncology ward in Iran: a cross-sectional study. Cancer Chemother Pharmacol. 2013;71:1619–27. - PubMed
-
- Marusic S, Bacic-Vrca V, Obreli Neto PR, Franic M, Erdeljic V, Gojo-Tomic N. Actual drug–drug interactions in elderly patients discharged from internal medicine clinic: a prospective observational study. Eur J Clin Pharmacol. 2013;69:1717–24. - PubMed
-
- Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Exp Opin Drug Saf. 2012;11(1):83–94. - PubMed
-
- Varma MV, Pang KS, Isoherranen N, Zhao P. Dealing with the complex drug–drug interactions: towards mechanistic models. Biopharm Drug Dispos. 2015;36(2):71–92. - PubMed
-
- Bjornsson T, Pharmaceutical Research, Manufacturers of America (PhRMA) Drug Metabolism/Clinical Pharmacology Technical Working Group; FDA Center for Drug Evaluation and Research (CDER). The conduct of in vitro and in vivo drug-drug interaction studies: a pharmaceutical research and manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31(7):815–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials