ERβ mediates sex-specific protection in the App-NL-G-F mouse model of Alzheimer's disease
- PMID: 40302001
- PMCID: PMC12039102
- DOI: 10.1186/s13293-025-00711-w
ERβ mediates sex-specific protection in the App-NL-G-F mouse model of Alzheimer's disease
Abstract
Background: Menopausal loss of neuroprotective estrogen is thought to contribute to the sex differences in Alzheimer's disease (AD). Activation of estrogen receptor beta (ERβ) can be clinically relevant since it avoids the adverse systemic effects of ERα activation. However, very few studies have explored ERβ-mediated neuroprotection in AD, and no information on its contribution to the sex differences in AD exists. In the present study, we specifically explored the role of ERβ in mediating sex-specific protection against AD pathology in the AppNL-G-F knock-in mouse model of amyloidosis, and if surgical menopause (ovariectomy) modulates pathology in this model.
Methods: We treated male and female AppNL-G-F knock-in mice with the clinically relevant and selective ERβ agonist LY500307. A subset of the females was ovariectomized prior to treatment. Y-maze and contextual fear conditioning tests were used to assess memory performance, and biochemical assays such as qPCR, immunohistochemistry, Western blot, and multiplex immunoassays, were used to evaluate amyloid pathology.
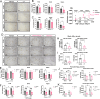
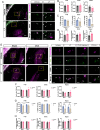
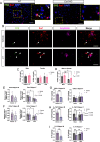
Results: We found that Female AppNL-G-F mice had higher soluble Aβ levels in cortex and hippocampus than males and more activated microglia. ERβ activation protected against amyloid pathology and cognitive decline in both male and female AppNL-G-F mice. Although ovariectomy increased soluble amyloid beta (Aβ) in cortex and insoluble Aβ in hippocampus, as well as sustained neuroinflammation after ERβ activation, it had otherwise limited effects on pathology. We further identified that ERβ did not alter APP processing, but rather exerted its protection at least partly via microglia activation in a sex-specific manner.
Conclusion: Combined, we provide new understanding to the sex differences in AD by demonstrating that ERβ protects against AD pathology differently in males and females, warranting reassessment of ERβ in combating AD.
Keywords: APP knock-in; Alzheimer’s disease; Amyloidosis; Estrogen receptor beta; Microglia; Sex differences; Sex hormone.
Plain language summary
About two-thirds of all Alzheimer’s disease (AD) patients are women. Although the reason for this sex difference is likely multifaceted, sex hormones are believed to be involved. The female sex hormone estrogen is known to mediate neuroprotection and loss of estrogen during the menopausal transition is believed to be a risk factor for AD. However, there is a gap in knowledge on how estrogenic neuroprotection occurs and if this neuroprotection is similar in men and women. In the current study, we specifically focused on one estrogen receptor, ERβ, and its role in mediating protection in a clinically relevant mouse model of AD and asked if there are any differences in this protection between male and female AD mice. Such information is of importance if proposing clinical trials targeting ERβ, which unlike targeting the ubiquitous estrogen receptor alpha (ERα), is not associated with adverse systemic effects. We found that ERβ activation indeed protects against amyloid plaque buildup and cognitive impairment in both males and females. Interestingly, this neuroprotection appeared to work differently in different brain regions and affected neuroinflammation and microglia immune cell function differently in males and females. Surgical menopause (ovariectomy) increased amyloid levels, which was counteracted by ERβ activation, and sustained high neuroinflammation but had otherwise limited effect on pathology. We provide the first study comparing ERβ-mediated protection on AD pathology in males and females, highlighting important sex differences that should be considered when proposing ERβ as a target to combat AD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures were performed in accordance with approved ethical permits (ethical approval ID 407 and ID 2199–2021, Linköping’s animal ethical board). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


Update of
-
ERβ mediates sex-specific protection in the App-NL-G-F mouse model of Alzheimer's disease.bioRxiv [Preprint]. 2024 Jul 23:2024.07.22.604543. doi: 10.1101/2024.07.22.604543. bioRxiv. 2024. Update in: Biol Sex Differ. 2025 Apr 29;16(1):29. doi: 10.1186/s13293-025-00711-w. PMID: 39091856 Free PMC article. Updated. Preprint.
References
-
- Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15(6):1050–9. 10.1097/gme.0b013e318174f155. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

