Inhibition of hippocampal mossy fiber plasticity and episodic memory by human Aβ oligomers is prevented by enhancing cAMP signaling in Alzheimer's mice
- PMID: 40302031
- PMCID: PMC12040739
- DOI: 10.1002/alz.70194
Inhibition of hippocampal mossy fiber plasticity and episodic memory by human Aβ oligomers is prevented by enhancing cAMP signaling in Alzheimer's mice
Abstract
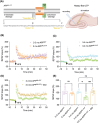
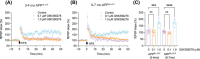
Introduction: Early episodic memory impairment in Alzheimer's disease (AD) is linked to synaptic dysfunction from amyloid β-protein oligomers (oAβ), particularly affecting the dentate gyrus mossy fiber-CA3 pathway. The APPNL-G-F mouse model exhibits early deficits in mossy fiber long-term potentiation (mf-LTP).
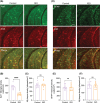
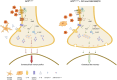
Methods: We administered the β-adrenergic receptor agonist isoproterenol (ISO) in vivo and phosphodiesterase type 4 inhibitor GSK356278 in vitro to assess their impact on mf-LTP and contextual fear memory. Fluorescence lifetime imaging (FLIM)-Förster resonance energy transfer (FRET) microscopy was used to visualize impaired and rescued cyclic adenosine monophosphate (cAMP) signaling in dentate gyrus neurons.
Results: ISO prevented mf-LTP impairment at 3-4 mo and improved memory by 7 mo. GSK356278 inhibited mf-LTP deficits in a dose-dependent manner. ISO also reduced hyperphosphorylation of synapsin I and microgliosis.
Discussion: These findings suggest that β-AR activation and phosphodiesterase 4 (PDE4) inhibition mitigate oAβ-induced memory deficits, supporting enhanced cAMP signaling as a therapeutic target for early AD.
Highlights: Early episodic memory deficits in AD linked to oAβ-induced synaptic dysfunction. Isoproterenol and GSK356278 improve mossy fiber-LTP and fear memory deficits. FLIM-FRET shows treatments restore cAMP signaling in dentate gyrus neurons. Isoproterenol reduces synapsin I hyperphosphorylation and microgliosis. Enhancing cAMP signaling may help mitigate early memory deficits in AD.
Keywords: APPNL‐G‐F knock‐in mice; Alzheimer's disease; Aβ oligomers; FLIM‐FRET; cAMP; episodic memory; long‐term potentiation; microgliosis; mossy fiber‐CA3 pathway; β‐adrenergic signaling.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
D.J.S. is a director of Prothena Biosciences and ad hoc consultant to Eisai and Roche. The other authors declare no competing interests. Author disclosures are available in the supporting information.
Figures




References
-
- Chapman PF, White GL, Jones MW, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271‐276. - PubMed
-
- Saito T, Matsuba Y, Mihira N, et al. Single App knock‐in mouse models of Alzheimer's disease. Nat Neurosci. 2014;17:661‐663. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

