Craving self-reports as outcome measures in drug addiction trials: A systematic review of ClinicalTrials.gov
- PMID: 40302057
- PMCID: PMC12426360
- DOI: 10.1111/add.70064
Craving self-reports as outcome measures in drug addiction trials: A systematic review of ClinicalTrials.gov
Abstract
Background and aims: The subjective experience of drug craving is characterized by an overwhelming urge to consume substances. Due to strong validity and ease of use, self-report measures are widely employed to assess substance-related motivational dynamics. Multi-item questionnaires are increasingly favored for capturing the multidimensional nature of craving, providing valuable insights for clinical care and relapse prediction. This review aimed to summarize craving self-report measurement tools registered in clinical trials and examine their methodological parameters.
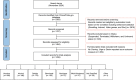
Methods: A search was conducted in November 2022 and updated in November 2024 using the same methodology on ClinicalTrials.gov for trials reporting drug craving as an outcome measure. Trials using craving measurement tools were screened and included.
Results: From 5383 initial trials, 1255 met eligibility criteria. Craving was reported as the only primary outcome measure in 14.6%, one of the primary outcomes in 21.3% and as secondary or exploratory in 64.1% of the studies. The most studied substances were nicotine (32.5% of studies) and alcohol (27.5%), followed by opioids (14.2%). Multi-item questionnaires were the most frequently used approach (55.4%), followed by single-item questionnaires (27.3%) to measure craving. Among 107 identified multi-item questionnaires, only 38 were used three or more times. The most common multi-item questionnaires were the Questionnaire on Smoking Urges (20%), Penn Alcohol Craving Scale (12.1%) and Alcohol Urge Questionnaire (9.8%). Most trials focused on evaluating phasic (now) craving (51.6%) over tonic (in a certain time-interval) craving (38%).
Conclusion: Craving, measured through self-reports, is increasingly targeted as a primary outcome measure in drug addiction trials registered on ClinicalTrials.gov. Craving self-reports are highly variable, underscoring the need for standardized tools to enhance comparability across studies.
Keywords: alcohol; clinical trial; craving; measurement tools; nicotine; self‐report questionnaire; substance use disorders; urge.
© 2025 The Author(s). Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
None.
Figures



References
-
- Hassani‐Abharian P, Mokri A, Ganjgahi H, Oghabian MA, Ekhtiari H. Validation for Persian versions of “desire for drug questionnaire” and “obsessive compulsive drug use scale” in heroin dependents. Arch Iran Med. 2016;19(9):659–665. - PubMed
-
- Kassel JD, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Adv Behav Res Ther. 1992;14(3):141–167. 10.1016/0146-6402(92)90006-A - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

