Improving Shared Decision-Making in Early Phase Clinical Trials and Palliative Care: A Prospective Study on the Impact of an Online Value Clarification Tool Intervention
- PMID: 40302152
- PMCID: PMC12041624
- DOI: 10.1002/pon.70168
Improving Shared Decision-Making in Early Phase Clinical Trials and Palliative Care: A Prospective Study on the Impact of an Online Value Clarification Tool Intervention
Abstract
Objectives: This study evaluated the impact of the OnVaCT intervention, a narrative-based Online Value Clarification Tool (OnVaCT), combined with communication training for oncologists, on shared decision-making (SDM) in discussions on potential early phase clinical trial participation and palliative care. These high-stakes decisions often challenge patients and oncologists in addressing patient values, a crucial component of SDM. We hypothesized that the intervention would improve oncologist-patient communication, specifically SDM application, and (consequently) reduce patient decisional conflict.
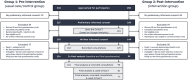
Methods: In this prospective, multicentre pre-post clinical study, patients completed two surveys, and their recorded consultations on early phase clinical trials and palliative care were assessed by independent coders. Pre-intervention patients received usual care, while post-intervention patients used the OnVaCT. Oncologists underwent communication training between study phases. Endpoints included decisional conflict (primary), the extent to which oncologists, patients and relatives participate in SDM, consultation length, and patient decisions (secondary).
Results: Decisional conflict (p = 0.394) did not differ between pre-test (n = 116, M = 30.0, SD = 16.9) and post-test (n = 99, M = 29.4, SD = 15.2). Oncologists significantly increased their SDM application post-intervention (p < 0.001; n = 129, M = 38.5, SD = 12.6) compared to pre-intervention (n = 163, M = 28.8, SD = 9.2), particularly when the OnVaCT was discussed. Other outcomes, including consultation length, remained stable.
Conclusions: The OnVaCT intervention enhanced SDM and supported value-based discussions, without prolonging consultations. Further research should explore whether additional implementation efforts could reduce decisional conflict and the intervention's potential impact on other patient-centred outcomes. Some decisions, however, may inherently involve unresolved conflict.
Keywords: cancer; decisional conflict; early phase clinical trials; oncology; online tool; patient‐provider communication; shared decision‐making; value clarification.
© 2025 The Author(s). Psycho‐Oncology published by John Wiley & Sons Ltd.
Conflict of interest statement
C.C.D.v.d.R. has received the grant from Dutch Cancer Society for the conduct of the study; and grants from the Netherlands Organization for Health Research and Development during the conduct of the study and a personal fee for consultancy from Lilly Nederland BV (via her institution), both outside the submitted work. M.P.L. reports grants from Astellas Pharma BV, Janssen Cilag BV, Sanofi Aventis Netherlands BV, Merck Sharp and Dohme BV, and payment or honoraria from Amgen, Janssen Cilag, Bayer, Servier, Roche, INCa, Pfizer, Astellas, Astra Zeneca, Merck, Novartis and Julius Clinical (all outside the submitted work) and is currently employed by Amgen Inc. The other authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical