Impact of Nirsevimab on RSV and Non-RSV Severe Respiratory Infections in Hospitalized Infants
- PMID: 40302169
- PMCID: PMC12041130
- DOI: 10.1111/irv.70105
Impact of Nirsevimab on RSV and Non-RSV Severe Respiratory Infections in Hospitalized Infants
Abstract
Background: Nirsevimab, a monoclonal antibody providing passive immunity against RSV infections in infants, was introduced in Spain in October 2023 for children under 6 months and those born during the epidemic season. This study aimed to compare the clinical and virological characteristics of respiratory infections in hospitalized infants before and after nirsevimab introduction.
Methods: We carried out a prospective study across two hospitals in Madrid during the 2022-2023 and 2023-2024 epidemic seasons. The study included infants under 12 months of age that were hospitalized with lower respiratory tract infections (LRTIs). Clinical, epidemiological, and virological data were analyzed and compared between the periods before and after the introduction of nirsevimab, as well as according to whether the infants had received this preventive treatment.
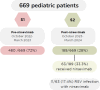
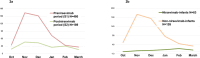
Results: A total of 669 infants were included: 480 from October 2022 to March 2023 (S1) and 189 from October 2023 to March 2024 (S2). Respiratory infection-related admissions decreased by 62.5% in S2, with a 74.5% reduction in ICU admissions. RSV-related admissions decreased by 78%, HMPV by 36.6%, and adenovirus by 69.5%. Infants in S2 were older (p = 0.001) and had shorter hospital stays (p < 0.001) than in S1. Of 63 (33%) infants in S2 who received nirsevimab, 11 (17%) were diagnosed with RSV. High-flow oxygen use was less frequent among RSV patients treated with nirsevimab (p = 0.002).
Conclusions: Nirsevimab introduction was significantly associated with reduced hospitalizations and severity of RSV and other respiratory infections. Its use was associated with fewer admissions and reduced need for intensive care, especially in RSV-infected infants but also in HMPV and adenovirus-infected infants.
Keywords: bronchiolitis; nirsevimab; respiratory infections; respiratory syncytial virus.
© 2025 The Author(s). Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Universal administration of nirsevimab in infants: an analysis of hospitalisations and paediatric intensive care unit admissions for RSV-associated lower respiratory tract infections.Eur J Pediatr. 2025 May 16;184(6):345. doi: 10.1007/s00431-025-06125-5. Eur J Pediatr. 2025. PMID: 40377714 Free PMC article.
-
Respiratory syncytial virus-related lower respiratory tract infection hospitalizations in infants receiving nirsevimab in Galicia (Spain): the NIRSE-GAL study.Eur J Pediatr. 2025 May 2;184(5):321. doi: 10.1007/s00431-025-06151-3. Eur J Pediatr. 2025. PMID: 40314706 Free PMC article.
-
Introduction of nirsevimab in Catalonia, Spain: description of the incidence of bronchiolitis and respiratory syncytial virus in the 2023/2024 season.Eur J Pediatr. 2024 Dec;183(12):5181-5189. doi: 10.1007/s00431-024-05779-x. Epub 2024 Sep 28. Eur J Pediatr. 2024. PMID: 39340677 Free PMC article.
-
Nirsevimab: Alleviating the burden of RSV morbidity in young children.J Paediatr Child Health. 2024 Oct;60(10):489-498. doi: 10.1111/jpc.16643. Epub 2024 Aug 16. J Paediatr Child Health. 2024. PMID: 39150043 Review.
-
Nirsevimab for the prevention of respiratory syncytial virus disease in children. Statement of the Spanish Society of Paediatric Infectious Disease (SEIP).An Pediatr (Engl Ed). 2023 Oct;99(4):257-263. doi: 10.1016/j.anpede.2023.09.006. Epub 2023 Sep 22. An Pediatr (Engl Ed). 2023. PMID: 37743207 Review.
Cited by
-
Respiratory syncytial virus prophylaxis with nirsevimab: impact beyond hospitalisations.Lancet Reg Health Eur. 2025 Jun 11;55:101349. doi: 10.1016/j.lanepe.2025.101349. eCollection 2025 Aug. Lancet Reg Health Eur. 2025. PMID: 40575468 Free PMC article. No abstract available.
References
-
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection,” Pediatrics 134, no. 2 (2014): 415–420. - PubMed
-
- Jones J. M., Fleming‐Dutra K. E., Prill M. M., et al., “Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices ‐ United States, 2023,” MMWR. Morbidity and Mortality Weekly Report 72 (2023): 920–925. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

