Propidium Monoazide Integrated With qPCR Enables Rapid and Universal Detection of Infectious Porcine Reproductive and Respiratory Syndrome Viruses
- PMID: 40303043
- PMCID: PMC12017026
- DOI: 10.1155/tbed/6250851
Propidium Monoazide Integrated With qPCR Enables Rapid and Universal Detection of Infectious Porcine Reproductive and Respiratory Syndrome Viruses
Abstract
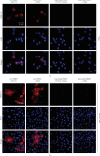
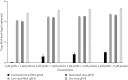
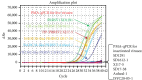
Infectious porcine reproductive and respiratory syndrome virus (PRRSV) causes PRRS, but noninfectious PRRSV cannot. PCR and ELISA are commonly used for PRRSV detection but they cannot discriminate PRRSV infectivity. Virus isolation is a gold standard to determine virus infectivity. However, it is time-consuming. Therefore, we developed a propidium monoazide (PMA) qPCR assay for rapid and universal detection of infectious PRRSV in this study. After comparing the inactivation efficacies of distinct disinfectants, ultraviolet (UV) light, and heat, heat at 72°C for 15 min was determined as an effective strategy for PRRSV inactivation, which was confirmed by virus isolation and immunofluorescence assay (IFA) detection. In addition, PMA pretreatment parameters were optimized, including PMA concentration (5 μM), PMA binding time (25 min), PMA binding temperature (37°C), and photolysis time (25 min). The optimal concentration of primers and probes adapted from our previous study was redetermined. The optimized PMA-qPCR assay exhibited satisfied specificity, sensitivity, and reproducibility. Furthermore, the new PMA-qPCR was applied on the detection of 270 clinical samples (including 57 environmental feces, 177 lungs, 33 lymph nodes [LN], and 3 sera) and compared with previously developed qPCR. Eighty samples were qPCR positive, while only 63 samples were PMA-qPCR positive. No virus could be isolated in the 17 qPCR-positive but PMA-qPCR-negative clinical samples; meanwhile, PRRSV could be isolated in representative PMA-qPCR-positive samples, supporting that only live PRRSV isolates in distinct samples could be detected by this PMA-qPCR assay. In conclusion, this study provides the first PMA-qPCR assay for rapid and universal detection of infectious PRRSV, offering an alternative and effective method for PRRSV diagnosis, prevention, and control.
Keywords: application; infectious virus detection; porcine reproductive and respiratory syndrome virus (PRRSV); propidium monoazide qPCR (PMA-qPCR).
Copyright © 2024 Wenhao Qi et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Propidium monoazide (PMA) and ethidium bromide monoazide (EMA) improve DNA array and high-throughput sequencing of porcine reproductive and respiratory syndrome virus identification.J Virol Methods. 2015 Sep 15;222:182-91. doi: 10.1016/j.jviromet.2015.06.014. Epub 2015 Jun 27. J Virol Methods. 2015. PMID: 26129867 Free PMC article.
-
Detection and typing of highly pathogenic porcine reproductive and respiratory syndrome virus by multiplex real-time rt-PCR.PLoS One. 2012;7(6):e38251. doi: 10.1371/journal.pone.0038251. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768042 Free PMC article.
-
Development of a Multiplex RT-qPCR Method for the Identification and Lineage Typing of Porcine Reproductive and Respiratory Syndrome Virus.Int J Mol Sci. 2024 Dec 8;25(23):13203. doi: 10.3390/ijms252313203. Int J Mol Sci. 2024. PMID: 39684913 Free PMC article.
-
Development of universal and quadruplex real-time RT-PCR assays for simultaneous detection and differentiation of porcine reproductive and respiratory syndrome viruses.Transbound Emerg Dis. 2019 Nov;66(6):2271-2278. doi: 10.1111/tbed.13276. Epub 2019 Jul 7. Transbound Emerg Dis. 2019. PMID: 31233656
-
[Review: diagnostic methods for the detection of porcine reproductive and respiratory syndrome virus (PRRSV) infections].Tijdschr Diergeneeskd. 2006 Aug 15;131(16):566-72. Tijdschr Diergeneeskd. 2006. PMID: 17007245 Review. Dutch.
Cited by
-
Evaluation of Torquetenovirus (TTV) Particle Integrity Utilizing PMAxx™.Int J Mol Sci. 2025 Jul 7;26(13):6542. doi: 10.3390/ijms26136542. Int J Mol Sci. 2025. PMID: 40650319 Free PMC article.
References
-
- Walker P. J., Siddell S. G., Lefkowitz E. J., et al. Changes to Virus Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2019) Archives of Virology . 2019;164(9):2417–2429. doi: 10.1007/s00705-019-04306-w. - DOI - PubMed
-
- Collins J. E., Benfield D. A., Christianson W. T., et al. Isolation of Swine Infertility and Respiratory Syndrome Virus (Isolate ATCC VR-2332) in North America and Experimental Reproduction of the Disease in Gnotobiotic Pigs. Journal of Veterinary Diagnostic Investigation . 1992;4(2):117–126. doi: 10.1177/104063879200400201. - DOI - PubMed
-
- Meng X. J., Paul P. S., Halbur P. G., Lum M. A. Phylogenetic Analyses of the Putative M (ORF 6) and N (ORF 7) Genes of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Implication for the Existence of Two Genotypes of PRRSV in the U.S.A. and Europe. Archives of Virology . 1995;140(4):745–755. doi: 10.1007/BF01309962. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

