Galleria mellonella Invertebrate Model Mirrors the Pathogenic Potential of Mycoplasma alligatoris within the Natural Host
- PMID: 40303151
- PMCID: PMC12017031
- DOI: 10.1155/2024/3009838
Galleria mellonella Invertebrate Model Mirrors the Pathogenic Potential of Mycoplasma alligatoris within the Natural Host
Abstract
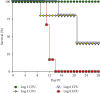
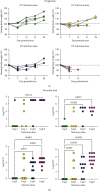
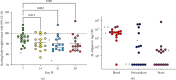
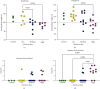
Most mycoplasmal infections result in chronic, clinically silent disease. In direct contrast, Mycoplasma alligatoris elicits a fulminant, multisystem disease in the natural host, Alligator mississippiensis (American alligator). The goals of the study were to better understand the disease in the natural host and to determine if the invertebrate model G. mellonella could serve as a surrogate alternate host. The survival of alligators infected intratracheally was dose dependent (p=0.0003), ranging from no mortality (102 CFU) to 100% mortality (108 CFU), with 60% mortality at the 104 and 105 CFU infectious dose. Microbial load in blood, joints, and brain was dose dependent, regardless of whether alligators were infected intratracheally or intravenously (p < 0.002). Weight loss was similarly impacted (p < 0.001). Experimental infection of the invertebrate Galleria mellonella mirrored the result in the natural host. In a dose response infection study, both larval survival curves and successful pupation curves were significantly different (p ≤ 0.0001) and dose dependent. Infected insects did not emerge as moths (p < 0.0001). Here, we describe the first study investigating G. mellonella as a surrogate model to assess the pathogenic potential of M. alligatoris. G. mellonella survival was dose dependent and impacted life stage outcome.
Copyright © 2024 Alexandra M. Burne et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Experimental inoculation of broad-nosed caimans (Caiman latirostris) and Siamese crocodiles (Crocodylus siamensis) with Mycoplasma alligatoris.J Zoo Wildl Med. 2001 Jun;32(2):196-201. doi: 10.1638/1042-7260(2001)032[0196:EIOBNC]2.0.CO;2. J Zoo Wildl Med. 2001. PMID: 12790420
-
Seroprevalence of Mycoplasma alligatoris among free-ranging alligators (Alligator mississippiensis) in Florida--2003.J Zoo Wildl Med. 2005 Jun;36(2):340-1. doi: 10.1638/04-029.1. J Zoo Wildl Med. 2005. PMID: 17323582
-
Detection of antibodies to a pathogenic mycoplasma in American alligators (Alligator mississippiensis), broad-nosed Caimans (Caiman latirostris), and Siamese crocodiles (Crocodylus siamensis).J Clin Microbiol. 2001 Jan;39(1):285-92. doi: 10.1128/JCM.39.1.285-292.2001. J Clin Microbiol. 2001. PMID: 11136785 Free PMC article.
-
Galleria mellonella as a screening tool to study virulence factors of Aspergillus fumigatus.Virulence. 2021 Dec;12(1):818-834. doi: 10.1080/21505594.2021.1893945. Virulence. 2021. PMID: 33682618 Free PMC article. Review.
-
Microbiology's next top model: Galleria in the molecular age.Pathog Dis. 2021 Feb 19;79(2):ftab006. doi: 10.1093/femspd/ftab006. Pathog Dis. 2021. PMID: 33476383 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

