Biomimetic piezoelectric hydrogel system for energy metabolism reprogramming in spinal cord injury repair
- PMID: 40303325
- PMCID: PMC12036890
- DOI: 10.7150/thno.108329
Biomimetic piezoelectric hydrogel system for energy metabolism reprogramming in spinal cord injury repair
Abstract
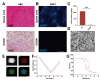
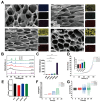
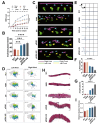
Rationale: Spinal cord injury (SCI) leads to limited regenerative capacity and severe energy deficiency in the injury microenvironment. This study aimed to develop a biomimetic piezoelectric hydrogel system that could recapitulate the native tissue microenvironment while enabling wireless physical regulation for SCI repair. Methods: A piezoelectric hydrogel was fabricated by integrating K0.5Na0.5NbO3 (KNN) nanoparticles with porous decellularized spinal cord matrix gel (pDG). The hydrogel's effects on vascular endothelial cell migration, neural stem cell differentiation, and ATP synthesis were evaluated in vitro. RNA sequencing was performed to identify key regulatory pathways. The therapeutic efficacy was assessed in a rat model of spinal cord hemisection, examining motor function and angiogenesis. Results: The piezoelectric hydrogel demonstrated excellent biocompatibility and significantly enhanced vascular endothelial cell and neural cell migration. Under ultrasonic stimulation, the hydrogel promoted neural stem cell differentiation into neurons more effectively than control hydrogels. The piezoelectric stimulation increased ATP synthesis and calcium ion flux, activating the Ca2+/Camk2b/PGC-1α signaling axis. In vivo studies showed that implantation of the piezoelectric hydrogel combined with ultrasound stimulation significantly improved motor function recovery and promoted angiogenesis. Conclusion: The piezoelectric hydrogel system presents an effective strategy for SCI repair through energy metabolism reprogramming and demonstrates promising potential in neural tissue engineering applications.
Keywords: ATP; neural regeneration; neural stem cells; piezoelectric hydrogel; spinal cord injury repair.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Neural-enhancing PRP/Alg/GelMA triple-network hydrogel for neurogenesis and angiogenesis after spinal cord injury via PI3K/AKT/mTOR signaling pathway.Theranostics. 2025 Mar 3;15(9):3837-3861. doi: 10.7150/thno.109091. eCollection 2025. Theranostics. 2025. PMID: 40213674 Free PMC article.
-
Bioactive Peptide Hydrogel Scaffold with High Fluidity, Thermosensitivity, and Neurotropism in 3D Spatial Structure for Promoted Repair of Spinal Cord Injury.Small. 2025 Jan;21(2):e2406990. doi: 10.1002/smll.202406990. Epub 2024 Nov 8. Small. 2025. PMID: 39513226
-
Injectable Hydrogel Loaded with CDs and FTY720 Combined with Neural Stem Cells for the Treatment of Spinal Cord Injury.Int J Nanomedicine. 2024 May 8;19:4081-4101. doi: 10.2147/IJN.S448962. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38736654 Free PMC article.
-
Multidimensional exploration of hydrogels as biological scaffolds for spinal cord regeneration: mechanisms and future perspectives.Front Bioeng Biotechnol. 2025 Apr 23;13:1576524. doi: 10.3389/fbioe.2025.1576524. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40336551 Free PMC article. Review.
-
Leveraging the Physicochemical Attributes of Biomimetic Hydrogel Nanocomposites in Stem Cell Differentiation.Biomacromolecules. 2024 Dec 9;25(12):7543-7562. doi: 10.1021/acs.biomac.4c00779. Epub 2024 Sep 15. Biomacromolecules. 2024. PMID: 39277809 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

