Noninvasive DWI tracking of hiPSCs differentiation into RTECs in AKI recovery via the KSP promoter-mediated AQP1 strategy
- PMID: 40303334
- PMCID: PMC12036887
- DOI: 10.7150/thno.109826
Noninvasive DWI tracking of hiPSCs differentiation into RTECs in AKI recovery via the KSP promoter-mediated AQP1 strategy
Abstract
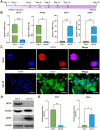
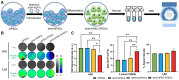
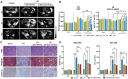
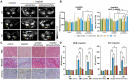
Rationale: Human-induced pluripotent stem cells (hiPSCs) exhibit great potential in the treatment of acute kidney injury (AKI), and their targeted differentiation into renal tubular epithelial cells (RTECs) is directly involved in the repair of the injured tissue. However, their differentiation during treatment is difficult to evaluate noninvasively. Methods: Aquaporin 1 (AQP1) can alter the dispersion of water molecules at the cellular level and thus can be detected with high sensitivity by diffusion-weighted imaging (DWI). In this study, a kidney-specific promoter (KSP-cadherin)-driven AQP1 overexpression lentivirus (KSP-AQP1) was constructed and used for tracking the differentiation of hiPSCs in vivo. Then, two AKI animal models were used to identify the feasibility of KSP-AQP1 for in vivo tracking of the differentiation of hiPSCs into RTECs. Results: We utilized KSP-positive and KSP-negative cells to examine the in vitro specificity of KSP-AQP1. It was found that only the KSP-positive cells showed a substantial expression of AQP1, accompanied by a significant variation in both the diffusion-weighted imaging (DWI) signal intensity (SI) and the apparent diffusion coefficient (ADC) values. whereas KSP-AQP1-transduced KSP-negative cells had no apparent SI and ADC changes. DWI results suggested that after the hiPSCs transplantation in vivo, the KSP-AQP1-pretransduced hiPSCs group exhibited a significantly decreased SI and increased ADC value when compared with the hiPSCs-treated and untreated AKI kidneys. In addition, the AQP1-mediated differences in DWI SI and ADC value between the KSP-AQP1-pretransduced hiPSCs group and hiPSCs group were confirmed by analysis of the KSP transcriptional activity using co-expressed exogenous flag gene mCherry. Conclusions: This study successfully developed a method for tracking the differentiation of hiPSCs into RTECs in vivo during the treatment of AKI using a KSP-regulated AQP1 overexpression strategy.
Keywords: acute kidney injury; aquaporin 1; diffusion-weighted imaging; human-induced pluripotent stem cells; tracking of differentiation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

