Extracellular vesicles play a central role in linking podocyte injury to mesangial activation in glomerular disease
- PMID: 40303345
- PMCID: PMC12036872
- DOI: 10.7150/thno.110034
Extracellular vesicles play a central role in linking podocyte injury to mesangial activation in glomerular disease
Abstract
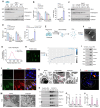
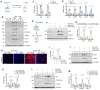
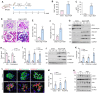
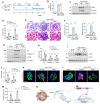
Background: Podocyte injury leading to proteinuria is the primary feature of a vast majority of glomerular diseases, while mesangial cell activation is the hallmark of glomerulosclerosis. Whether and how these two events are connected remains elusive. In this study, we investigated the role of extracellular vesicles (EVs) in linking podocyte injury to mesangial activation in glomerular disease. Methods: EVs were characterized by nanoparticle tracking analysis and electron microscopy. Differentially expressed proteins from podocyte-derived EVs were analyzed by protein microarray. The role and mechanism by which EVs-packaged sonic hedgehog (Shh) mediates mesangial cell activation were investigated in vitro and in vivo. Results: An increased production of EVs in mouse podocytes (MPC5) was observed after injury induced by angiotensin II (Ang II). Shh and N-Shh were identified as major constituents of the proteins encapsulated in EVs isolated from Ang II-treated MPC5 cells (Ang II-EVs). In vitro, Ang II-EVs induced the activation and proliferation of rat mesangial cells (HBZY-1), whereas inhibition of EV secretion with dimethyl amiloride, depletion of EVs from conditioned media or knockdown of Shh expression abolished the ability of Ang II-EVs to induce HBZY-1 activation. In vivo, intravenous injection of Ang II-EVs exacerbated glomerulosclerosis, which was negated by hedgehog inhibitor. Furthermore, blocking EV secretion also ameliorated glomerulosclerosis in mouse model of glomerular disease. Conclusions: These findings suggest that podocyte injury can cause mesangial cell activation and glomerulosclerosis by releasing Shh-enriched EVs. Therefore, strategies targeting EVs may be a novel way to ameliorate proteinuric kidney disease.
Keywords: Glomerulosclerosis; Shh; extracellular vesicles; podocyte-mesangial communication; proteinuria.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Kidney Disease. Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–S276. - PubMed
-
- Chadban SJ, Atkins RC. Glomerulonephritis. Lancet. 2005;365:1797–806. - PubMed
-
- Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. - PubMed
-
- D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63:809–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

