Serine supplementation suppresses hypoxia-induced pathological retinal angiogenesis
- PMID: 40303351
- PMCID: PMC12036884
- DOI: 10.7150/thno.105299
Serine supplementation suppresses hypoxia-induced pathological retinal angiogenesis
Abstract
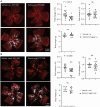
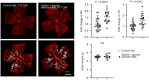
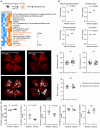
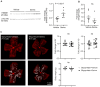
Rationale: Pathological retinal angiogenesis with irregular and fragile vessels (also termed neovascularization, a response to hypoxia and dysmetabolism) is a leading cause of vision loss in all age groups. This process is driven in part through the energy deficiency in retinal neurons. Sustaining neural retinal metabolism with adequate nutrient supply may help prevent vision-threatening neovascularization. Low circulating serine levels are associated with neovascularization in macular telangiectasia and altered serine/glycine metabolism has been suggested in retinopathy of prematurity. We here explored the role of serine metabolism in suppressing hypoxia-driven retinal neovascularization using oxygen-induced retinopathy (OIR) mouse model. Methods: We administered wild-type C57BL/6J OIR pups with systemic serine or provided the nursing dam with a serine/glycine-deficient diet during the relative hypoxic phase, followed by analysis of retinal vasculature at postnatal (P) 17, the time of peak neovascularization. Retinas from P17 OIR pups with either systemic serine supplementation or vehicle control were subjected to metabolomics, lipidomics, proteomics, and single-cell RNA sequencing. To validate the role of mitochondrial fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS) in mediating serine protection against OIR, we treated OIR pups with inhibitors to block FAO or OXPHOS along with either serine or vehicle. The potential transcriptional mediator and pro-angiogenic signals were validated by western blotting. Results: Systemic serine supplementation reduced retinal neovascularization, while maternal dietary serine/glycine deficiency exacerbated it. FAO was essential in mediating serine protective effects, and serine supplementation increased levels of phosphatidylcholine, the most abundant phospholipids in the retina. Serine treatment a) increased the abundance of proteins involved in OXPHOS in retinas, b) increased the expression of mitochondrial respiration-related genes, and c) decreased the expression of pro-angiogenic genes in rod photoreceptor cluster. Serine suppression of retinal neovascularization was dependent on mitochondrial energy production. High mobility group box 1 protein (HMGB1) was identified as a potential key mediator of serine suppression of pro-angiogenic signals in hypoxic retinas. Conclusions: Our findings suggest that serine supplementation may serve as a potential therapeutic approach for neovascular eye diseases by enhancing retinal mitochondrial metabolism and lipid utilization, suppressing the key drivers of uncontrolled angiogenesis.
Keywords: angiogenesis; neovascularization; retina; retinopathy of prematurity; serine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C. et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33. e1. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

