The role of complement in the immunopathogenesis of acetylcholine receptor antibody-positive generalized myasthenia gravis: bystander or key player?
- PMID: 40303417
- PMCID: PMC12037622
- DOI: 10.3389/fimmu.2025.1526317
The role of complement in the immunopathogenesis of acetylcholine receptor antibody-positive generalized myasthenia gravis: bystander or key player?
Abstract
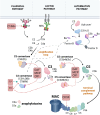
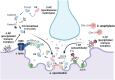
The complement system is a key component of the innate immune system. In antiacetylcholine receptor (AChR) antibody-positive (Ab+) generalized myasthenia gravis (MG), complement activation has long been considered a principal driver of pathology. Understanding the role of complement in AChR-Ab+ generalized MG has gained increasing importance in recent years, as anticomplement drugs have been approved for clinical use or are undergoing phase II/III clinical trials. This review aims to discuss recent and previous findings on the role of complement in AChR-Ab+ MG pathology, including its interaction with pathogenic antibodies and mechanisms beyond the classical pathway activation.
Keywords: anti-acetylcholine receptor antibodies; complement system; membrane attack complex; myasthenia gravis; therapy.
Copyright © 2025 Michailidou, Patsiarika, Kesidou, Boziki, Parisis, Bakirtzis, Chroni and Grigoriadis.
Conflict of interest statement
AP is an employee of AstraZeneca Rare Disease. EC has participated in advisory meetings and satellite symposia organized by Merck, Sanofi Genzyme, Biogen, Genesis Pharma, Teva Pharmaceuticals, UCB Pharma S.A., Lavipharm, ITF Hellas, Medison Pharma-Argenx, Alexion-Astra Zeneca, and Takeda, and has received unrestricted research grants from Genesis Pharma, Merck, Novartis, and Lavipharm. NG has received honoraria, travel support, consultancy, and lecture fees from Biogen Idec, Novartis, TEVA, Bayer, Merck Serono, Genesis Pharma, Sanofi-Genzyme, Celgene, ELPEN, ROCHE, Alexion-Astra Zeneca, UCB, Medison, Lavipharn, and Pharmaserve, as well as research grants from Biogen Idec, Novartis, TEVA, Merck Serono, Genesis Pharma, Sanofi-Genzyme, and ROCHE. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

