Plant resistance inducer AMHA enhances antioxidant capacities to promote cold tolerance by regulating the upgrade of glutathione S-transferase in tea plant
- PMID: 40303428
- PMCID: PMC12038892
- DOI: 10.1093/hr/uhaf073
Plant resistance inducer AMHA enhances antioxidant capacities to promote cold tolerance by regulating the upgrade of glutathione S-transferase in tea plant
Abstract
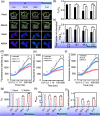
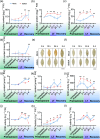
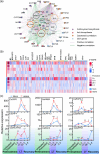
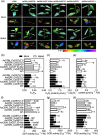
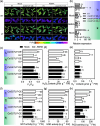
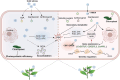
Plant resistance inducers represent an alternative strategy that mitigate stress-induced damage in plants. Previously, 2-amino-3-methylhexanoic acid (AMHA), a novel natural plant resistance inducer, was shown to significantly bolster cold tolerance, thermotolerance, and pathogen resistance in plants. However, the intricate mechanisms underlying AMHA's response to cold stress remain elusive. Thus, we investigated the physiological and transcriptomic analyses of AMHA pretreatment on tea plant to determine its substantial role of AMHA under cold stress. The results showed that pretreatment with 100 nM AMHA effectively mitigated the detrimental effects of cold stress on photosynthesis and growth. Furthermore, differentially expressed genes were identified through RNA-seq during pretreatment, cold stress, and 2 days of recovery. These genes were mainly enriched in pathways related to flavonoid/anthocyanin, carotenoid, and ascorbic acid-glutathione (AsA-GSH) cycle, including GST (encoding glutathione S-transferase). Potential regulatory relationships between the identified genes and transcription factors were also established. Antisense oligodeoxynucleotide-silencing and overexpression experiments revealed that CsGSTU7 enhances cold resistance by maintaining redox homeostasis. In conclusion, our study suggests that antioxidant-related signaling molecules play a critical role in the signaling cascades and transcriptional regulation mediating AMHA-induced cold-stress resistance in tea plant.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Guo X, Liu D, Chong K. Cold signaling in plants: insights into mechanisms and regulation. J Integr Plant Biol. 2018;60:745–56 - PubMed
-
- Kidokoro S, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022;27:922–35 - PubMed
-
- Li X, Ahammed GJ, Li Z. et al. Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci Hortic. 2017;230:155–60
-
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30 - PubMed
-
- Ron Mittler SIZ, Fichman Y, Van Breusegem. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol. 2022;23:663–79 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

