Root-specific expression of CsNPF2.3 is involved in modulating fluoride accumulation in tea plant (Camellia sinensis)
- PMID: 40303432
- PMCID: PMC12038894
- DOI: 10.1093/hr/uhaf072
Root-specific expression of CsNPF2.3 is involved in modulating fluoride accumulation in tea plant (Camellia sinensis)
Abstract
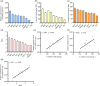
Fluoride (F) is a nonessential but potentially harmful element for plants, especially when present in excess. The tea plant is known for its ability to hyperaccumulate F from the soil and eventually accumulates in the leaves; however, how the tea plant transports F to the leaves remains unclear. Here, we found that Se can significantly decrease the transport efficiency of F from root to leaf. Therefore, RNA-Sequencing was performed on tea roots cotreated with selenite and fluoride, and then we isolated a plasma membrane-localized F transporter CsNPF2.3 from tea plant roots and examined its role in transport of F in tea plants. The results showed that CsNPF2.3 exhibited F transport activity when heterologously expressed in yeast. Expression pattern analysis revealed that CsNPF2.3 is expressed in epidermal cells, cortex cells, and xylem parenchyma cells in roots. Overexpression of CsNPF2.3 in tea roots significantly increased F content in the root, stem, and leaf, and enhanced the transport efficiency of F from root to leaf. Furthermore, in nine tea cultivars, CsNPF2.3 expression in the root was significantly positively correlated with F content in the leaf and root, and the transport efficiency of F from root to leaf. Altogether, these findings suggest that CsNPF2.3 was involved in uptake and transport of F in tea plants.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Selenium treatment modulates fluoride distribution and mitigates fluoride stress in tea plant (Camellia sinensis (L.) O. Kuntze).Environ Pollut. 2020 Dec;267:115603. doi: 10.1016/j.envpol.2020.115603. Epub 2020 Sep 7. Environ Pollut. 2020. PMID: 33254693
-
Positron-emitting tracer imaging of fluoride transport and distribution in tea plant.J Sci Food Agric. 2020 Jun;100(8):3554-3559. doi: 10.1002/jsfa.10367. Epub 2020 Mar 22. J Sci Food Agric. 2020. PMID: 32124449
-
Aluminum relieves fluoride stress through stimulation of organic acid production in Camellia sinensis.Physiol Mol Biol Plants. 2020 Jun;26(6):1127-1137. doi: 10.1007/s12298-020-00813-2. Epub 2020 May 2. Physiol Mol Biol Plants. 2020. PMID: 32549678 Free PMC article.
-
Fluoride absorption, transportation and tolerance mechanism in Camellia sinensis, and its bioavailability and health risk assessment: a systematic review.J Sci Food Agric. 2021 Jan 30;101(2):379-387. doi: 10.1002/jsfa.10640. Epub 2020 Aug 8. J Sci Food Agric. 2021. PMID: 32623727
-
Theanine metabolism and transport in tea plants (Camellia sinensis L.): advances and perspectives.Crit Rev Biotechnol. 2023 May;43(3):327-341. doi: 10.1080/07388551.2022.2036692. Epub 2022 Apr 17. Crit Rev Biotechnol. 2023. PMID: 35430936 Review.
References
-
- Wang M, Wang H, Lei G. et al. Current progress on fluoride occurrence in the soil environment: sources, transformation, regulations and remediation. Chemosphere. 2023;341:139901 - PubMed
-
- De A, Mridha D, Ray I. et al. Fluoride exposure and probabilistic health risk assessment through different agricultural food crops from fluoride endemic Bankura and Purulia Districts of West Bengal, India. Front Environ Sci. 2021;9:713148
-
- Koblar A, Tavčar G, Ponikvar-Svet M. Fluoride in teas of different types and forms and the exposure of humans to fluoride with tea and diet. Food Chem. 2012;130:286–90
-
- Das S, De Oliveira LM, Da Silva E. et al. Fluoride concentrations in traditional and herbal teas: health risk assessment. Environ Pollut. 2017;231:779–84 - PubMed
-
- Rodrigues AA, Vasconcelos-Filho SC, Mendes GC. et al. Fluoride in simulated rain affects the morphoanatomy and physiology of Eugenia dysenterica (Mart.) DC. Ecol Indic. 2017;82:189–95
LinkOut - more resources
Full Text Sources

