Bacillus velezensis TB918 mitigates garlic dry rot disease by forming consortia with Pseudomonas in the rhizosphere and bulb
- PMID: 40303477
- PMCID: PMC12037484
- DOI: 10.3389/fmicb.2025.1567108
Bacillus velezensis TB918 mitigates garlic dry rot disease by forming consortia with Pseudomonas in the rhizosphere and bulb
Abstract
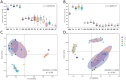
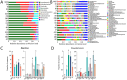
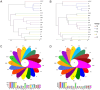
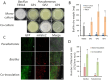
Garlic dry rot (GDR), primarily caused by Fusarium proliferatum, is a significant postharvest disease that leads to substantial economic losses. Our previous research demonstrated that supplementing Bacillus-based biocontrol formulations with sucrose could boost its efficiency in protecting plants by building a hostile rhizomicrobiome for destructive soil-borne pathogens. B. velezensis TB918, previously isolated from pepper rhizosphere soil, exhibited a strong in vitro antifungal effect on Fusarium. In this study, we conducted a field experiment to investigate the efficacy of B. velezensis TB918 in controlling GDR, and explored the changes in microbial communities in garlic plants and rhizosphere soil following the application of TB918 with or without sucrose supplementation. Using 16S rRNA and ITS amplicon sequencing, we found that the introduction of TB918 significantly increased the abundance of Pseudomonas in garlic rhizosphere, especially when combined with sucrose. Three Pseudomonas strains were isolated from garlic tissues and rhizosphere soil treated with TB918 and sucrose, among which the GP2 strain exhibited antagonistic effects against pathogen ad planta. Co-culture and colonization assays showed that TB918 facilitated the biofilm formation of Pseudomonas strain by forming consortia. Interestingly, the abundance of potentially non-pathogenic Fusarium concentricum also increased, suggesting a potential niche exclusion effect. Our results demonstrated that TB918 in combination with sucrose effectively reduced the incidence of GDR during storage. This study provides valuable insights into the use of biocontrol agents and sucrose to modulate the garlic microbial community and suppress soil-borne pathogens.
Keywords: Bacillus velezensis TB918; Fusarium; biocontrol; garlic dry rot; rhizosphere microbiota.
Copyright © 2025 Shi, Sun, Sun, Wang, Li, Wu and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bastian M., Heymann S., Jacomy M. (2009). Gephi: an open source software for exploring and manipulating networks. ICWSM 3, 361–362. doi: 10.1609/icwsm.v3i1.13937 - DOI
LinkOut - more resources
Full Text Sources

