Nanomaterial-Based Drug Delivery Systems Targeting Functional Cells for Osteoarthritis Treatment: Mechanisms, Challenges and Future Prospects
- PMID: 40303574
- PMCID: PMC12039932
- DOI: 10.2147/IJN.S518935
Nanomaterial-Based Drug Delivery Systems Targeting Functional Cells for Osteoarthritis Treatment: Mechanisms, Challenges and Future Prospects
Abstract
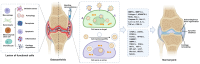
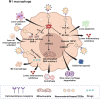
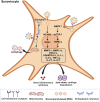
Osteoarthritis (OA) represents a chronic joint disease characterized by articular cartilage degeneration, synovial inflammation, and subchondral bone erosions. Functional cells in OA mainly include macrophages, synoviocytes, chondrocytes, and mesenchymal stem cells. These cells can secrete cytokines and non-coding RNAs and exosomes and interact with each other to coregulate the progression of OA. Some nanomaterial-based drug delivery systems (DDSs) surface ligands can alleviate OA by targeting receptors on the surface of functional cells. Meanwhile, other nanomaterial-based DDSs, whose surfaces are masked by the cell membranes or extracellular vesicles of these functional cells, treat OA by targeting and attacking the diseased site. When ligand-modified nanomaterials target specific functional cells to treat OA, the functional cells are attacked. Functional cells become attackers, similar to arrows, when their cell membranes or extracellular vesicles are modified into nanomaterials to deliver drugs for OA treatment. An increasing number of studies have been conducted on nanomaterial-based DDS-targeted functional cells for the treatment of OA, but none has summarized the corresponding research progress and mechanism of action. In this review, the related references on the treatment of osteoarthritis with nanomaterial-based DDSs targeting functional cells have been included, and how a variety of functional cells can be engineered into nanomaterial-based DDSs serving as targets or arrows to treat OA has been summarised for the first time, providing a new idea and method for the targeted treatment of OA.
Keywords: chondrocytes; macrophages; mesenchymal stem cells; nanomaterial-based drug delivery systems; osteoarthritis; synoviocytes.
© 2025 Kong et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

