Lymphocyte signatures correspond to clinical phenotypes in autoimmune limbic encephalitis
- PMID: 40303600
- PMCID: PMC12038345
- DOI: 10.1093/braincomms/fcaf156
Lymphocyte signatures correspond to clinical phenotypes in autoimmune limbic encephalitis
Abstract
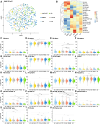
Autoimmune limbic encephalitis is an inflammatory condition confined to the limbic system of the brain that is deemed to be due to a dysregulated immune response. However, the exact pathophysiological mechanisms remain elusive. Diagnosis of autoimmune limbic encephalitis currently relies on clinical consensus criteria. However, diagnostic workup can be challenging, potentially delaying treatment initiation associated with poor clinical outcomes. We retrospectively identified 640 patients (81 autoimmune limbic encephalitis, 148 relapsing-remitting multiple sclerosis, 197 Alzheimer's disease, 67 frontotemporal dementia, 37 temporal lobe epilepsy with hippocampal sclerosis and 110 somatic symptom disorder patients). Applying multidimensional flow-cytometry together with novel computational approaches, we analysed the peripheral blood and cerebrospinal fluid immune cell profiles at different disease stages and performed correlations with clinical parameters (i.e. neuropsychological performance, EEG and MRI). We were able to identify a shared immune signature of autoimmune limbic encephalitis showing similarities in adaptive B and T cell response with other inflammatory central nervous system diseases and in T cell patterns with neurodegenerative disorders. Antibody-negative autoimmune limbic encephalitis showed a pronounced T cell response in peripheral blood similar to temporal lobe epilepsy and hippocampal sclerosis and neurodegenerative disorders differentiating from antibody-positive autoimmune limbic encephalitis and classical inflammatory central nervous system diseases with regard to B and plasma cell response. Longitudinal immune cell phenotyping in autoimmune limbic encephalitis revealed dynamic changes over time mainly affecting the innate, B and plasma cell compartment. Correlation analysis indicated associations between the baseline immune cell profile, especially lymphocytes, and neuropsychological performance, as well as EEG and MRI abnormalities. Applying novel computational approaches, we found that multidimensional flow cytometry together with routine CSF parameters could reliably distinguish autoimmune limbic encephalitis from controls and clinical differential diagnoses. Incorporation of multidimensional flow cytometry parameters showed superior discriminatory ability compared with CSF routine parameters alone. Taken together, autoimmune limbic encephalitis is characterized by a B and T cell dominated intrathecal immune-cell signature corresponding to changes reported in the brain parenchyma and showing similarities with classical inflammatory central nervous system diseases and neurodegenerative disorders. Incorporating clinical parameters and applying novel computational approaches, we could show that multidimensional flow cytometry might be a beneficial complement to the established diagnostic workup of autoimmune limbic encephalitis promoting early diagnosis and facilitating outcome prediction to enhance individualized treatment regimes.
Keywords: differential diagnoses; immune cells; limbic encephalitis; multidimensional flow cytometry.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
S.K. reports research funding from Biogen and has received speakers’ honoraria from Eisai, Jazz Pharma and UCB. The remaining authors report no conflicts of interest.
Figures





References
LinkOut - more resources
Full Text Sources
