Clinical Features of Anti-Tuberculosis Drug-Induced Liver Injury and Risk Factors for Severe Cases: A Retrospective Study in China
- PMID: 40303606
- PMCID: PMC12039843
- DOI: 10.2147/IDR.S519211
Clinical Features of Anti-Tuberculosis Drug-Induced Liver Injury and Risk Factors for Severe Cases: A Retrospective Study in China
Abstract
Background: Anti-tuberculosis drug-induced liver injury (ATB-DILI) is a common adverse reaction associated with tuberculosis (TB) treatment, significantly impacting treatment adherence and therapeutic outcomes. However, large-scale studies on hospitalized patients in China remain limited.
Purpose: To characterize the clinical features and liver injury patterns in hospitalized TB patients with ATB-DILI and to identify risk factors associated with severe ATB-DILI.
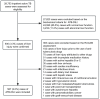
Methods: We retrospectively reviewed 28,753 hospitalized TB patients at Beijing Chest Hospital from 2014 to 2023. ATB-DILI was diagnosed in 567 patients (2.0%) based on serum biochemical criteria and causality assessment. Demographic, clinical, and laboratory data were analyzed to characterize liver injury types and identify risk factors for severe cases. Subgroup analyses based on liver injury patterns were performed to further evaluate the association between age and severe ATB-DILI.
Results: Overall, 567 cases with ATB-DILI (2.0%) were analyzed. Hepatocellular injury was the most common type (71.4%), followed by cholestatic (13.8%) and mixed (14.8%) injury patterns. Most patients (68.4%) were asymptomatic and diagnosed via routine biochemical monitoring; jaundice occurred in 18.2%. Patients with hepatocellular damage were significantly younger, while those with cholestatic injury were older (p < 0.001). Severe ATB-DILI occurred in 46 patients (8.1%), with advanced age (≥60 years) identified as an independent risk factor (OR = 2.45, 95% CI: 1.33-4.52, p = 0.004). Subgroup analysis showed that this association between age and severe ATB-DILI was significant in the hepatocellular injury type (unadjusted OR = 3.59, 95% CI: 1.61-8.02, p = 0.002), while no statistically significant association was observed in cholestatic or mixed types, which may reflect limited statistical power in these subgroups.
Conclusion: Routine liver function monitoring and age-specific risk assessment are essential for early identification and management of ATB-DILI in hospitalized TB patients.
Keywords: anti-tuberculosis drugs; drug-induced liver injury; hepatotoxicity; risk factors; severe liver injury.
© 2025 Zhang et al.
Conflict of interest statement
The authors declare that there are no commercial or financial relationships that could be perceived as a potential conflict of interest in relation to this research.
Figures

Similar articles
-
[Guidelines for diagnosis and management of drug-induced liver injury caused by anti-tuberculosis drugs (2024 version)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Nov 12;47(11):1069-1090. doi: 10.3760/cma.j.cn112147-20240614-00338. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 39497389 Chinese.
-
Urine metabolomics and microbiome analyses reveal the mechanism of anti-tuberculosis drug-induced liver injury, as assessed for causality using the updated RUCAM: A prospective study.Front Immunol. 2022 Nov 22;13:1002126. doi: 10.3389/fimmu.2022.1002126. eCollection 2022. Front Immunol. 2022. PMID: 36483548 Free PMC article.
-
Clinical risk factors for moderate and severe antituberculosis drug-induced liver injury.Front Pharmacol. 2024 Jul 23;15:1406454. doi: 10.3389/fphar.2024.1406454. eCollection 2024. Front Pharmacol. 2024. PMID: 39108745 Free PMC article.
-
[Progress in novel biomarkers of anti-tuberculosis drug-induced liver injury].Zhonghua Jie He He Hu Xi Za Zhi. 2024 May 12;47(5):469-474. doi: 10.3760/cma.j.cn112147-20230915-00161. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38706071 Review. Chinese.
-
An Update on Drug-induced Liver Injury.J Clin Exp Hepatol. 2012 Sep;2(3):247-59. doi: 10.1016/j.jceh.2012.05.002. Epub 2012 Sep 21. J Clin Exp Hepatol. 2012. PMID: 25755441 Free PMC article. Review.
References
-
- World Health Organization. Global tuberculosis report, Geneva, Switzerland, 2024.
-
- Naidoo K, Hassan-Moosa R, Mlotshwa P, et al. High rates of drug-induced liver injury in people living with HIV coinfected with tuberculosis (TB) irrespective of antiretroviral therapy timing during antituberculosis treatment: results from the starting antiretroviral therapy at three points in TB trial. Clin Infect Dis. 2020;70(12):2675–2682. doi:10.1093/cid/ciz732 - DOI - PMC - PubMed
-
- Sharifzadeh M, Rasoulinejad M, Valipour F, Nouraie M, Vaziri S. Evaluation of patient-related factors associated with causality, preventability, predictability and severity of hepatotoxicity during antituberculosis [correction of antituberclosis] treatment. Pharmacol Res. 2005;51(4):353–358. doi:10.1016/j.phrs.2004.10.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources

