Relations between neurometabolism and clinical biomarkers in patients with metabolic disease
- PMID: 40303609
- PMCID: PMC12037510
- DOI: 10.3389/fnins.2025.1547010
Relations between neurometabolism and clinical biomarkers in patients with metabolic disease
Abstract
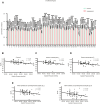
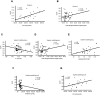
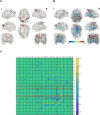
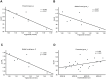
The global prevalence of metabolic diseases, including hypertension, type 2 diabetes mellitus (T2DM), gout, and obesity, has significantly increased over the past two decades. The brain plays a central role in regulating both human behavior and metabolism. Understanding the potential connections among these metabolic diseases and the involvement of the brain in their progression presents an intriguing and critical area of research. In this study, we analyzed PET-CT images and clinical biomarkers from 112 cases of hypertension, 56 cases of T2DM, 11 cases of obesity, and 14 cases of gout. Standardized uptake value ratios (SUVRs) were extracted from various brain regions using the Spatial-Normalization-of-Brain-PET-Images (SNBPI) software. The SUVRs were calculated using the standard methodology, where the mean standardized uptake value (SUV) of each region of interest (ROI) was divided by the mean SUV of the reference region, that is the whole cerebellum. The SNBPI tool was employed for intensity normalization. Partial correlation analysis was conducted to examine the relationships between SUVRs in different brain regions and clinical biomarkers, adjusting for sex, age, and BMI. Brain network metabolic connectivity was assessed using Permutation_IHEP software and visualized with BrainNet Viewer. Our results indicate that SUVRs in most brain regions were decreased in patients with hypertension or T2DM but increased in patients with obesity or gout. Specifically, SUVRs in brain regions associated with blood pressure were correlated with blood uric acid, creatinine, potassium, and apolipoprotein B. SUVRs in brain regions related to blood glucose were associated with blood triglycerides and cholinesterase. SUVRs in BMI-related brain regions correlated with blood urea nitrogen, aspartate aminotransferase, and alkaline phosphatase. SUVRs in brain regions associated with gout were correlated with fasting blood glucose, glutamic oxalacetic transaminase, total bilirubin, and alkaline phosphatase. Furthermore, brain network metabolic connectivity was reduced in patients with hypertension, T2DM, or obesity but increased in patients with gout. Our findings suggest that uric acid may negatively relate with blood pressure and glucose levels, while blood glucose and blood lipid levels may be positively correlated with each other. Gout appears distinct from other metabolic diseases and may offer a protective effect on brain function. The right superior parietal gyrus may be implicated in impaired renal function during the progression of hypertension. The left precentral gyrus and bilateral middle frontal gyri may relate to dyslipidemia and the potential development of atherosclerotic cardiovascular disease in patients with T2DM. In conclusion, our study highlights potential relationships among metabolic diseases and suggests the possible regulatory roles of specific brain regions in the progression of these conditions. These insights could pave the way for novel therapeutic strategies targeting brain metabolism in the management of metabolic diseases.
Keywords: PET-CT; T2DM; clinical biomarkers; gout; hypertension; obesity.
Copyright © 2025 Chen, Tan, Yin, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

