Biochemical evaluation of molecular parts for flavonoid production using plant synthetic biology
- PMID: 40303856
- PMCID: PMC12038446
- DOI: 10.3389/fpls.2025.1528122
Biochemical evaluation of molecular parts for flavonoid production using plant synthetic biology
Abstract
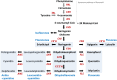
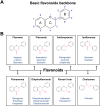
Among organisms on Earth, plants have the unique ability to produce a wide variety of biomolecules using soil nutrients, air, and solar energy. Therefore, plants are regarded as the most productive and cost-efficient bioreactors among living organisms. Flavonoids, a major group of secondary metabolites exclusively produced in plants, play crucial roles in plant physiology and have various effects on human health. Flavonoids are used in diverse industries such as the pharmaceutical, nutraceutical, and cosmetics industries. These compounds are typically extracted from specific plants that naturally produce large amounts of the target flavonoid for commercial production. However, with the increasing demand for flavonoids, efforts have been made to enhance flavonoid production using synthetic biology for sustainable production in microbes or plants. Synthetic biology has been utilized for plant metabolic engineering to reconstitute the biosynthetic pathways of target flavonoids at the whole-pathway level, thereby enhancing flavonoid production. For the most efficient flavonoid production using plant synthetic biology, first of all, optimized molecular parts and enzymes must be identified and selected. The best modules to produce the precursors and final target flavonoids can then be constructed using these optimized parts. In this review, we summarize the enzyme kinetics of natural and engineered molecular parts derived from different plant species and provide insight into the selection of molecular parts, design of devices, and reconstitution of pathways based on enzyme performance for sustainable flavonoid production using plant synthetic biology.
Keywords: enzyme activity; flavonoids; molecular parts; plant biofactory; plant synthetic biology.
Copyright © 2025 Lee, Lee, Park, Song and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Akashi T., Fukuchi-Mizutani M., Aoki T., Ueyama Y., Yonekura-akakibara K., Tanaka Y., et al. . (1999). Molecular cloning and biochemical characterization of a novel cytochrome P450, flavone synthase II, that catalyzes direct conversion of flavanones to flavones. Plant Cell Physiol. 40, 1182–1186. doi: 10.1093/oxfordjournals.pcp.a029505 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

