The TFRC as a prognostic biomarker and potential therapeutic target in cervical cancer: a preliminary study
- PMID: 40303995
- PMCID: PMC12037406
- DOI: 10.3389/fonc.2025.1523137
The TFRC as a prognostic biomarker and potential therapeutic target in cervical cancer: a preliminary study
Abstract
Background: Early detection and treatment of CIN or early-stage cervical cancer lead to better clinical outcomes compared to treating advanced-stage patients. Thus, specific biomarkers for the diagnosis and prognosis of CIN and early-stage cervical cancer should be urgently explored.
Methods: We analyzed tumor based on genes closely related to OS in the database with GSE63514, GSE7803, GSE9750 and TCGA data sets, the top 20 core genes were screened out. Notably, transferrin receptor (TFRC) emerged as a prioritized candidate due to its dual role in cellular iron homeostasis and oncogenic signaling. However, the exact role of TFRC in the development and progression of cervical cancer remains unclear. We then used various bioinformatics methods and mathematical models to analyze those data, aiming to investigate the clinical significance of TFRC in cervical cancer and illustrate its association with tumor immunity. In addition, the molecular function and mechanisms of TFRC were revealed by gene ontology, Kyoto Encyclopedia of Genes and Genomes, and gene set enrichment analysis. Immunohistochemistry was employed to assess TFRC protein expression in 19 cervical cancers, 16 HSILs and 15 normal cervical tissues.
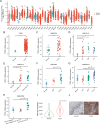
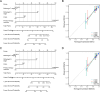
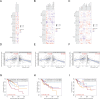
Results: TFRC was highly expressed in CESC in the TCGA and GSE9750 datasets. Meanwhile, the expression of TFRC was correlated with pathological stage, lymph node metastasis, malignant degree of cervical lesions and HPV infection status. Our analysis confirmed that TFRC expression was higher in CESC tissues compared to normal cervical tissues, and it was also elevated in HSIL relative to normal tissues, as determined by IHC staining. Increased TFRC expression was linked to decreased overall survival (OS) (p = 0.024), disease-specific survival (DSS) (p = 0.009), and progression-free interval (PFI) (p = 0.007) in CESC patients. In different clinical stages, pathological T stages, and pathological N stages, higher TFRC expression was significantly associated with worse survival for OS and DSS. We constructed a nomogram model, TFRC contributed significantly to the prognosis and exhibited good predictive power for the OS and the DSS. Finally, we confirmed that immunosuppression in cervical cancer is closely related to high TFRC expression.
Conclusions: TFRC exhibits significant diagnostic and prognostic value in cervical cancer.
Keywords: TFRC; bioinformatics; cervical cancer; cervical intraepithelial neoplasia; immune cell infiltration.
Copyright © 2025 Wang, An, Pang, Zhao, Xu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

