Daphnetin alleviates inflammation and promotes autophagy via the AMPK/mTOR pathway in gouty arthritis
- PMID: 40304008
- PMCID: PMC12037417
- DOI: 10.1002/ccs3.70011
Daphnetin alleviates inflammation and promotes autophagy via the AMPK/mTOR pathway in gouty arthritis
Abstract
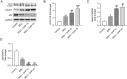
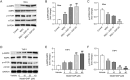
Gouty arthritis (GA) is an inflammatory disease resulting from monosodium urate (MSU) crystal deposition in joints and surrounding tissues. Daphnetin (DAP) is a coumarin derivative with potent anti-inflammatory activity. Nonetheless, whether DAP can protect against MSU-induced acute GA remains unclarified. In this study, C57BL/6 mice were injected intra-articularly with MSU crystal suspension to induce acute GA. THP-1 cells were stimulated with MSU to mimic the microenvironment of GA in vitro. Hematoxylin-eosin staining was conducted to observe the pathological changes in mouse synovial tissues. ELISA and RT-qPCR were employed for inflammatory cytokine level determination. Immunofluorescence staining was performed to estimate LC3 expression in THP-1 cells. Western blotting was used for protein expression analysis. The results showed that DAP pretreatment mitigated MSU-elicited ankle joint swelling and synovial damage in mice. Moreover, DAP hindered proinflammatory factor expression and promoted autophagy in MSU-stimulated GA mice and THP-1 cells. Mechanistically, DAP induced AMPK activation and mTOR inactivation. Blocking AMPK signaling counteracted DAP-mediated effects on inflammation and autophagy in MSU-stimulated THP-1 cells. In conclusion, DAP prevents MSU-elicited GA by alleviating inflammation and enhancing autophagy via AMPK/mTOR signaling transduction.
Keywords: autophagy; daphnetin; gouty arthritis; monosodium urate crystal.
© 2025 The Author(s). Journal of Cell Communication and Signaling published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Nobiletin ameliorates monosodium urate-induced gouty arthritis in mice by enhancing AMPK/mTOR-mediated autophagy to inhibit NF-κB/NLRP3 inflammasome activation.Immunol Lett. 2025 Aug;274:106982. doi: 10.1016/j.imlet.2025.106982. Epub 2025 Feb 16. Immunol Lett. 2025. PMID: 39965668
-
MicroRNA-142-3p facilitates inflammatory response by targeting ZEB2 and activating NF-κB signaling in gouty arthritis.Cell Cycle. 2022 Apr;21(8):805-819. doi: 10.1080/15384101.2022.2031678. Epub 2022 Mar 3. Cell Cycle. 2022. PMID: 35239453 Free PMC article.
-
DUSP1 Mitigates MSU-Induced Immune Response in Gouty Arthritis Reinforcing Autophagy.Front Biosci (Landmark Ed). 2024 Jun 20;29(6):222. doi: 10.31083/j.fbl2906222. Front Biosci (Landmark Ed). 2024. PMID: 38940057
-
Astilbin inhibited neutrophil extracellular traps in gouty arthritis through suppression of purinergic P2Y6 receptor.Phytomedicine. 2024 Jul 25;130:155754. doi: 10.1016/j.phymed.2024.155754. Epub 2024 May 17. Phytomedicine. 2024. PMID: 38820662
-
Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage.Arthritis Res Ther. 2019 Aug 27;21(1):193. doi: 10.1186/s13075-019-1974-z. Arthritis Res Ther. 2019. PMID: 31455356 Free PMC article.
References
-
- Catalán, Laura , Carceller María Carmen, Terencio María Carmen, Alcaraz María José, Ferrándiz María Luisa, and Montesinos María Carmen. 2024. “Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase‐1 Activation and Upregulation of Nrf2 Expression.” International Journal of Molecular Sciences 25(5): 2752. 10.3390/ijms25052752. - DOI - PMC - PubMed
-
- Cheng, J.‐Juan , Ma X.‐Dong, Ai G.‐Xiang, Yu Q.‐Xia, Chen X.‐Ying, Yan Fang, Li Yu‐Cui, Xie J.‐Hui, Su Zi‐Ren, and Xie Q.‐Feng. 2022. “Palmatine Protects against MSU‐Induced Gouty Arthritis via Regulating the NF‐κB/NLRP3 and Nrf2 Pathways.” Drug Design, Development and Therapy 16: 2119–2132. 10.2147/dddt.s356307. - DOI - PMC - PubMed
-
- Wang, Y. , Wei Li, Tianhan Zhang, Runmei Wang, Tao Wang, Qingyun Xie, and Meng Wei. 2023. “Resveratrol Alleviates MSU‐Induced Gouty Arthritis in Rats through Inhibition of HIF‐1α‐ and NLRP3‐Derived IL‐1β Secretion in Macrophages.” Cell Mol Biol (Noisy‐le‐grand) 69(7): 28–34. 10.14715/cmb/2023.69.7.5. - DOI - PubMed
-
- Wang, Hao , Chen Si‐ting, Ding X.‐jie, Kuai Le, Hua Liang, Li Xin, Wang Yi‐fei, et al. 2024. “Efficacy and Safety of Huzhang Granule, a Compound Chinese Herbal Medicine, for Acute Gouty Arthritis: A Double‐Blind, Randomized Controlled Trial.” J Integr Med 22(3): 270–278. 10.1016/j.joim.2024.03.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

