S-acylation in apoptotic and non-apoptotic cell death: a central regulator of membrane dynamics and protein function
- PMID: 40304073
- PMCID: PMC12203964
- DOI: 10.1042/BST20253012
S-acylation in apoptotic and non-apoptotic cell death: a central regulator of membrane dynamics and protein function
Abstract
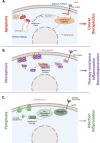
Protein lipidation is a collection of important post-translational modifications that modulate protein localization and stability. Protein lipidation affects protein function by facilitating interactions with cellular membranes, changing the local environment of protein interactions. Among these modifications, S-acylation has emerged as a key regulator of various cellular processes, including different forms of cell death. In this mini-review, we highlight the role of S-acylation in apoptosis and its emerging contributions to necroptosis and pyroptosis. While traditionally associated with the incorporation of palmitic acid (palmitoylation), recent findings indicate that other fatty acids can also participate in S-acylation, expanding its functional repertoire. In apoptosis, S-acylation influences the localization and function of key regulators such as Bcl-2-associated X protein and other proteins modulating their role in mitochondrial permeabilization and death receptor signaling. Similarly, in necroptosis, S-acylation of mixed lineage kinase domain-like protein (MLKL) with palmitic acid and very long-chain fatty acids enhances membrane binding and membrane permeabilization, contributing to cell death and inflammatory responses. Recent studies also highlight the role of S-acylation in pyroptosis, where S-acylated gasdermin D facilitates membrane localization and pore assembly upon inflammasome activation. Blocking palmitoylation has shown to suppress pyroptosis and cytokine release, reducing inflammatory activity and tissue damage in septic models. Collectively, these findings underscore S-acylation as a shared and important regulatory mechanism across cell death pathways affecting membrane association of key signaling proteins and membrane dynamics, and offer insights into the spatial and temporal control of protein function.
Keywords: S-acylation; apoptosis; cell death; necroptosis; palmitoylation; pyroptosis.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts associated with the manuscript.
Figures

Similar articles
-
ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis).Front Cell Infect Microbiol. 2019 Nov 26;9:406. doi: 10.3389/fcimb.2019.00406. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31850239 Free PMC article. Review.
-
Straight A's: protein acylation in the S-activation and autophagic degradation of NOD-like receptors.Biochem Soc Trans. 2025 Jul 4:BST20253026. doi: 10.1042/BST20253026. Online ahead of print. Biochem Soc Trans. 2025. PMID: 40613780 Free PMC article.
-
The Multifaceted Role of Calcium Signaling in Regulated Necrosis.Biomolecules. 2025 Jun 11;15(6):854. doi: 10.3390/biom15060854. Biomolecules. 2025. PMID: 40563494 Free PMC article. Review.
-
Fat traffic control: S-acylation in axonal transport.Mol Pharmacol. 2025 Jun;107(6):100039. doi: 10.1016/j.molpha.2025.100039. Epub 2025 Apr 16. Mol Pharmacol. 2025. PMID: 40349611 Free PMC article. Review.
-
[Programmed cell death in paramyxovirus infection].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025 May 25;54(3):399-410. doi: 10.3724/zdxbyxb-2024-0512. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40394914 Free PMC article. Review. Chinese.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

